Learnings from the Relation between the Number of Forward and Reverse Reactions (Transfer Cycles) Required to Converge to Equilibrium and the Ratio of the Forward to the Reverse Rate Constants in Simple Chemical Reactions
- PMID: 33458457
- PMCID: PMC7807462
- DOI: 10.1021/acsomega.0c05130
Learnings from the Relation between the Number of Forward and Reverse Reactions (Transfer Cycles) Required to Converge to Equilibrium and the Ratio of the Forward to the Reverse Rate Constants in Simple Chemical Reactions
Abstract
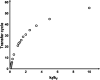
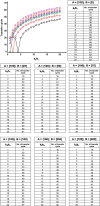
In simple, reversible, chemical reactions of the type A ⇋ B, chemical equilibrium is related to chemical kinetics via the equality between the equilibrium constant and the ratio of the forward to the reverse rate-constant, i.e., K eq = k f/k r, where K eq is the equilibrium constant and k f and k r denote the rate constants for the forward (A → B) and reverse (B → A) reactions, respectively. We review and examine the relation between the number of forward and reverse reactions required to take place for the aforementioned system to reach equilibrium and the ratio of the forward to the reverse rate constant. Each cycle of reactants becoming products and the products becoming reactants is defined as the transfer cycle (TC). Therefore, we underscore the relation between the number of TCs required for the system to equilibrate and k f/k r. We also vary the initial concentrations of the reactants and products to examine their dependency of the relation between the number of TCs required to reach equilibrium and k f/k r. The data reveal a logarithmic growth-type relation between the number of TCs required for the system to achieve equilibrium and k f/k r. The results of this relation are discussed in the context of several scenarios that populate the trajectory. We conclude by introducing students and researchers in the area of chemistry and biochemistry to physical phenomena that relate the initial concentrations of the reactants and products and k f/k r to the number of TCs necessary for the system to equilibrate.
© 2020 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Chang R.Chemistry, 10th ed.; Chang R., Ed.; McGraw Hill: New York, 2010; pp 614–657.
-
- Banerjee A. C. Teaching chemical equilibrium and thermodynamics in undergraduate general chemistry classes. J. Chem. Educ. 1995, 72, 879–881. 10.1021/ed072p879. - DOI
-
- Cloonan C. A.; Nichol C. A.; Hutchinson J. S. Understanding chemical reaction kinetics and equilibrium with interlocking building blocks. J. Chem. Educ. 2011, 88, 1400–1403. 10.1021/ed1010773. - DOI
-
- Quílez J. From chemical forces to chemical rates: A historical/philosophical foundation for the teaching of chemical equilibrium. Sci. & Educ. 2009, 18, 1203–1251. 10.1007/s11191-006-9048-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources

