Systematic Evaluations of Doxorubicin-Induced Toxicity in Rats Based on Metabolomics
- PMID: 33458487
- PMCID: PMC7807767
- DOI: 10.1021/acsomega.0c04677
Systematic Evaluations of Doxorubicin-Induced Toxicity in Rats Based on Metabolomics
Abstract
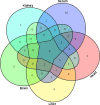
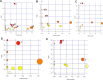
Doxorubicin (DOX) is widely used to treat solid tumors, but its use is limited by its severe cardiotoxicity, nephrotoxicity, hepatotoxicity, and neurotoxicity. Metabolomic studies on DOX-induced toxicity are mainly focused on alterations in the heart and kidney, but systematic research on multiple matrices (serum, heart, liver, brain, and kidney) is rare. Thus, in our study, gas chromatography-mass spectrometry analysis of main targeted tissues (serum, heart, liver, brain, and kidney) was used to systemically evaluate the toxicity of DOX. Multivariate analyses, including orthogonal projections to the latent structure and t-test, revealed 21 metabolites in the serum, including cholesterol, d-glucose, d-lactic acid, glycine, l-alanine, l-glutamic acid, l-isoleucine, l-leucine, l-proline, l-serine, l-tryptophan, l-tyrosine, l-valine, MG (0:0/18:0/0:0), MG (16:0/0:0/0:0), N-methylphenylethanolamine, oleamide, palmitic acid, pyroglutamic acid, stearic acid, and urea. In the heart, perturbed metabolites included 3-methyl-1-pentanol, cholesterol, d-glucose, d-lactic acid, glycerol, glycine, l-alanine, l-valine, MG (16:0/0:0/0:0), palmitic acid, phenol, propanoic acid, and stearic acid. For the liver, DOX exposure caused alterations of acetamide, acetic acid, d-glucose, glycerol, l-threonine, palmitic acid, palmitoleic acid, stearic acid, and urea. In the brain, metabolic changes involved 2-butanol, carbamic acid, cholesterol, desmosterol, d-lactic acid, l-valine, MG (16:0/0:0/0:0), palmitic acid, and stearic acid. In the kidney, disturbed metabolites were involved in cholesterol, glycerol, glycine, l-alanine, MG (0:0/18:0/0:0), MG (16:0/0:0/0:0), and squalene. Complementary evidence by multiple matrices revealed disturbed pathways concerning amino acid metabolism, energy metabolism, and lipid metabolism. Our results may help to systematically elucidate the metabolic changes of DOX-induced toxicity and clarify the underlying mechanisms.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

