New Alkyne and Amine Linkers for Versatile Multiple Conjugation of Oligonucleotides
- PMID: 33458510
- PMCID: PMC7807750
- DOI: 10.1021/acsomega.0c05075
New Alkyne and Amine Linkers for Versatile Multiple Conjugation of Oligonucleotides
Abstract
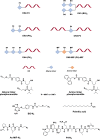
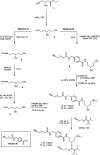
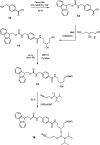
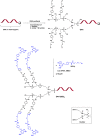
Oligonucleotide (ON) conjugates are increasingly important tools for various molecular diagnostics, nanotechnological applications, and for the development of nucleic acid-based therapies. Multiple labeling of ONs can further equip ON-conjugates and provide improved or additional tailored properties. Typically, the preparation of ON multiconjugates involves additional synthetic steps and/or manipulations in post-ON assembly. This report describes the simplified methodology allowing for multiple labeling of ONs on a solid support and is compatible with phosphodiester as well as phosphorothioate (PS) ONs. The current approach utilizes two novel alkyne- and amino-functionalized linker phosphoramidites that can be readily synthesized from a common aminodiol intermediate in three steps. The combination of new linkers provides orthogonal functionalities, which allow for multiple attachments of similar or varied moieties. The linkers are incorporated into ONs during automated solid-phase ON synthesis, and the conjugation with functional entities is achieved by either amide bond formation or by copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC). The versatility of the approach is demonstrated by the synthesis of 5'-site ON multiconjugates with small molecules, peptides, and fatty acids as well as in the preparation of an internal peptide-ON conjugate.
© 2020 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Copper-Catalyzed Huisgen 1,3-Dipolar Cycloaddition Tailored for Phosphorothioate Oligonucleotides.Curr Protoc Nucleic Acid Chem. 2020 Mar;80(1):e102. doi: 10.1002/cpnc.102. Curr Protoc Nucleic Acid Chem. 2020. PMID: 31884728
-
Efficient Conjugation to Phosphorothioate Oligonucleotides by Cu-Catalyzed Huisgen 1,3-Dipolar Cycloaddition.Bioconjug Chem. 2019 Jun 19;30(6):1622-1628. doi: 10.1021/acs.bioconjchem.9b00217. Epub 2019 May 14. Bioconjug Chem. 2019. PMID: 31067031
-
Attachment of Peptides to Oligonucleotides on Solid Support Using Copper(I)-Catalyzed Huisgen 1,3-Dipolar Cycloaddition.Methods Mol Biol. 2019;2036:165-171. doi: 10.1007/978-1-4939-9670-4_9. Methods Mol Biol. 2019. PMID: 31410796
-
Synthesis and Applications of Porphyrin-Biomacromolecule Conjugates.Front Chem. 2021 Nov 8;9:764137. doi: 10.3389/fchem.2021.764137. eCollection 2021. Front Chem. 2021. PMID: 34820357 Free PMC article. Review.
-
Glycoclusters on oligonucleotide and PNA scaffolds: synthesis and applications.Chem Soc Rev. 2013 Jun 7;42(11):4557-73. doi: 10.1039/c2cs35406c. Epub 2012 Dec 19. Chem Soc Rev. 2013. PMID: 23254681 Review.
Cited by
-
Aqueous Compatible Post-Synthetic On-Column Conjugation of Nucleic Acids Using Amino-Modifiers.Chembiochem. 2025 Jan 2;26(1):e202400643. doi: 10.1002/cbic.202400643. Epub 2024 Nov 13. Chembiochem. 2025. PMID: 39333054 Free PMC article.
-
Hydrophobic CPP/HDO conjugates: a new frontier in oligonucleotide-warheaded PROTAC delivery.RSC Med Chem. 2024 Sep 26;15(11):3695-703. doi: 10.1039/d4md00546e. Online ahead of print. RSC Med Chem. 2024. PMID: 39421539 Free PMC article.
-
Effective, Rapid, and Small-Scale Bioconjugation and Purification of "Clicked" Small-Molecule DNA Oligonucleotide for Nucleic Acid Nanoparticle Functionalization.Int J Mol Sci. 2023 Mar 2;24(5):4797. doi: 10.3390/ijms24054797. Int J Mol Sci. 2023. PMID: 36902228 Free PMC article.
-
Synthesis and stability studies of bicyclo[6.1.0]nonyne scaffolds for automated solid-phase oligonucleotide synthesis.RSC Adv. 2024 May 29;14(25):17406-17412. doi: 10.1039/d3ra08732h. eCollection 2024 May 28. RSC Adv. 2024. PMID: 38813131 Free PMC article.
-
Lipid and Peptide-Oligonucleotide Conjugates for Therapeutic Purposes: From Simple Hybrids to Complex Multifunctional Assemblies.Pharmaceutics. 2023 Jan 18;15(2):320. doi: 10.3390/pharmaceutics15020320. Pharmaceutics. 2023. PMID: 36839642 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

