Oral Bioavailability Comparison of Artemisinin, Deoxyartemisinin, and 10-Deoxoartemisinin Based on Computer Simulations and Pharmacokinetics in Rats
- PMID: 33458540
- PMCID: PMC7808142
- DOI: 10.1021/acsomega.0c05465
Oral Bioavailability Comparison of Artemisinin, Deoxyartemisinin, and 10-Deoxoartemisinin Based on Computer Simulations and Pharmacokinetics in Rats
Abstract
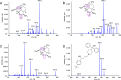
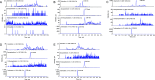
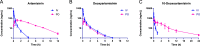
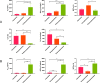
Deoxyartemisinin, a compound separated from Artemisinin annua L., shows anti-inflammatory and antiulcer activities. 10-Deoxoartemisinin is a novel compound with a strong antimalarial effect derivatized from artemisinin. Compared to the famous antimalarial natural compound artemisinin, deoxyartemisinin lacks the peroxide bridge structure, while 10-deoxoartemisinin remains this special peroxide bridge group but loses the 10-position keto group. To clarify their pharmacological differences, the absorption, distribution, metabolism, excretion (ADME) properties of artemisinin, deoxyartemisinin, and 10-deoxoartemisinin were first predicted using QikProp software. Also, their pharmacokinetic behaviors in rats were further evaluated by a rapid, sensitive, and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method after oral and intravenous administration of each compound, in which deoxyartemisinin and 10-deoxoartemisinin were first evaluated for their pharmacokinetics. All parameters about ADME properties calculated by software met the criteria and the ADME performance order was 10-deoxoartemisinin > deoxyartemisinin > artemisinin. The oral bioavailability of artemisinin was calculated to be 12.2 ± 0.832%, which was about 7 times higher than that of deoxyartemisinin (1.60 ± 0.317%). For 10-deoxoartemisinin, its bioavailability (26.1 ± 7.04%) was superior to artemisinin at a degree of more than twice. Considering their chemical structures, losing the peroxide bridge might decrease the absorption rate of deoxyartemisinin in the gastrointestinal tract, while retaining the peroxide bridge but losing the 10-position ketone might improve the bioavailability of 10-deoxoartemisinin.
© 2020 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Leeson P. D.; Empfield J. R. Reducing the risk of drug attrition associated with physicochemical properties. Annu. Rep. Med. Chem. 2010, 45, 393–407. 10.1016/S0065-7743(10)45024-1. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

