Exosomes from adipose-derived stem cells and application to skin wound healing
- PMID: 33458899
- PMCID: PMC7941238
- DOI: 10.1111/cpr.12993
Exosomes from adipose-derived stem cells and application to skin wound healing
Abstract
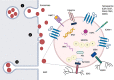
Skin wound healing is an intractable problem that represents an urgent clinical need. To solve this problem, a large number of studies have focused on the use of exosomes (EXOs) derived from adipose-derived stem cells (ADSCs). This review describes the mechanisms whereby ADSCs-EXOs regulate wound healing and their clinical application. In the wound, ADSCs-EXOs modulate immune responses and inflammation. They also promote angiogenesis, accelerate proliferation and re-epithelization of skin cells, and regulate collagen remodelling which inhibits scar hyperplasia. Compared with ADSCs therapeutics, ADSCs-EXOs have highly stability and are easily stored. Additionally, they are not rejected by the immune system and have a homing effect and their dosage can be easily controlled. ADSCs-EXOs can improve fat grafting and promote wound healing in patients with diabetes mellitus. They can also act as a carrier and combined scaffold for treatment, leading to scarless cutaneous repair. Overall, ADSCs-EXOs have the potential to be used in the clinic to promote wound healing.
Keywords: adipose-derived stem cells; angiogenesis; exosomes; inflammation; skin wound healing.
© 2021 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2014;22(1):23‐33. 10.1111/wrr.12135 - DOI - PubMed
-
- Orgill DP, Ogawa R. Discussion: the embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg. 2014;133(2):406‐407. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

