Can Some Anticancer Treatments Preserve the Ovarian Reserve?
- PMID: 33458904
- PMCID: PMC8176995
- DOI: 10.1002/onco.13675
Can Some Anticancer Treatments Preserve the Ovarian Reserve?
Abstract
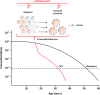
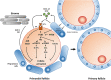
Background: Preventing premature ovarian failure (POF) is a major challenge in oncology. With conventional regimens, cytotoxicity-associated POF involves primordial follicles (PF) pool depletion by apoptosis or overactivation mechanisms, notably mediated by the ABL/TAp63 and PI3K/Akt/mTOR pathways. New anticancer treatments have been designed to target pathways implicated in tumor growth. Although concerns regarding fertility arise with these targeted therapies, we hypothesized that targeted therapies may exert off-tumor effects on PF that might delay POF. We provide an overview of evidence concerning these off-tumor effects on PF. Limitations and future potential implications of these findings are discussed.
Design: PubMed was searched by combining Boolean operators with the following keywords: fertility, ovarian, follicle, anti-tumoral, cancer, targeted, cytotoxic, and chemotherapy.
Results: Cisplatin-related PF apoptosis via the ABL/TAp63 pathway was targeted with a tyrosine kinase inhibitor, imatinib, in mice, but effects were recently challenged by findings on human ovarian xenografts in mice. In cyclophosphamide-treated mice, PI3K/Akt/mTOR pathway inhibition with mTOR inhibitors and AS101 preserved the PF pool. Proteasome and GSK3 inhibitors were evaluated for direct and indirect follicle DNA damage prevention. Surprisingly, evidence for cytotoxic drug association with PF pool preservation was found. We also describe selected non-anticancer molecules that may minimize gonadotoxicity.
Conclusion: Not all anticancer treatments are associated with POF, particularly since the advent of targeted therapies. The feasibility of associating a protective drug targeting PF exhaustion mechanisms with cytotoxic treatments should be evaluated, as a way of decreasing the need for conventional fertility preservation techniques. Further evaluations are required for transfer into clinical practice.
Implications for practice: Anticancer therapies are associated with infertility in 10%-70% of patients, which is the result of primordial follicles pool depletion. Alone or associated with gonadotoxic treatments, some targeted therapies may exert favorable off-targets effects on the primordial follicle pool by slowing down their exhaustion. Current evidence of these effects relies on murine models or human in vitro models. Evaluation of these protective strategies in humans is challenging; however, if these results are confirmed with clinical and biological data, it not only could be a new approach to female fertility preservation but also would change standard fertility strategies.
Keywords: Cancer; Chemotherapy; Fertility preservation; Premature ovarian failure; Targeted therapy.
© 2021 AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Salama M, Anazodo A, Woodruff TK. Preserving fertility in female patients with hematological malignancies: A multidisciplinary oncofertility approach. Ann Oncol 2019;30:1760–1775. - PubMed
-
- Poirot C, Abirached F, Prades M et al. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet 2012;379:588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

