Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery
- PMID: 33458958
- PMCID: PMC7995055
- DOI: 10.1002/adhm.202001812
Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery
Abstract
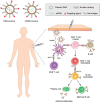
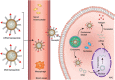
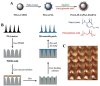
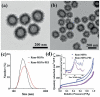
Nucleic acid vaccines are a method of immunization aiming to elicit immune responses akin to live attenuated vaccines. In this method, DNA or messenger RNA (mRNA) sequences are delivered to the body to generate proteins, which mimic disease antigens to stimulate the immune response. Advantages of nucleic acid vaccines include stimulation of both cell-mediated and humoral immunity, ease of design, rapid adaptability to changing pathogen strains, and customizable multiantigen vaccines. To combat the SARS-CoV-2 pandemic, and many other diseases, nucleic acid vaccines appear to be a promising method. However, aid is needed in delivering the fragile DNA/mRNA payload. Many delivery strategies have been developed to elicit effective immune stimulation, yet no nucleic acid vaccine has been FDA-approved for human use. Nanoparticles (NPs) are one of the top candidates to mediate successful DNA/mRNA vaccine delivery due to their unique properties, including unlimited possibilities for formulations, protective capacity, simultaneous loading, and delivery potential of multiple DNA/mRNA vaccines. This review will summarize the many varieties of novel NP formulations for DNA and mRNA vaccine delivery as well as give the reader a brief synopsis of NP vaccine clinical trials. Finally, the future perspectives and challenges for NP-mediated nucleic acid vaccines will be explored.
Keywords: DNA; mRNA; nanoparticles; nucleic acid; vaccine delivery; vaccines.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

