Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy
- PMID: 33459443
- PMCID: PMC8048856
- DOI: 10.1002/stem.3322
Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy
Erratum in
-
Erratum.Stem Cells. 2021 Dec;39(12):E6. doi: 10.1002/stem.3464. Stem Cells. 2021. PMID: 34866289 Free PMC article. No abstract available.
-
Correction to: Cartilage Endplate Stem Cells Inhibit Intervertebral Disc Degeneration by Releasing Exosomes to Nucleus Pulposus Cells to Activate Akt/Autophagy.Stem Cells. 2023 Apr 25;41(4):415. doi: 10.1093/stmcls/sxac092. Stem Cells. 2023. PMID: 36821781 No abstract available.
Abstract
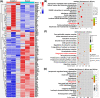
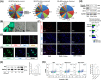
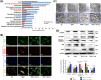
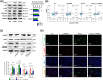
Degeneration of the cartilage endplate (CEP) induces intervertebral disc degeneration (IVDD). Nucleus pulposus cell (NPC) apoptosis is also an important exacerbating factor in IVDD, but the cascade mechanism in IVDD is not clear. We investigated the apoptosis of NPCs and IVDD when stimulated by normal cartilage endplate stem cell (CESC)-derived exosomes (N-Exos) and degenerated CESC-derived exosomes (D-Exos) in vitro and in vivo. Tert-butyl hydroperoxide (TBHP) was used to induce inflammation of CESCs. The bioinformatics differences between N-Exos and D-Exos were analyzed using mass spectrometry, heat map, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. NPC apoptosis was examined using TUNEL staining. The involvement of the AKT and autophagy signaling pathways was investigated using the signaling inhibitor LY294002. Magnetic resonance imaging, Western blotting, and immunofluorescence staining were used to evaluate the therapeutic effects of N-Exos in rats with IVDD. TBHP effectively induced inflammation and the degeneration of CEP in rat. N-Exos were more conducive to autophagy activation than D-Exos. The apoptotic rate of NPCs decreased obviously after treatment with N-Exos compared to D-Exos. N-Exos inhibited NPCs apoptosis and attenuated IVDD in rat via activation of the AKT and autophagy pathways. These results are the first findings to confirm that CEP delayed the progression of IVDD via exosomes. The therapeutic effects of N-Exos on NPC apoptosis inhibition and the slowing of IVDD progression were more effective than D-Exos due to activation of the PI3K/AKT/autophagy pathway, which explained the increase in the incidence of IVDD after inflammation of the CEP.
Keywords: apoptosis; autophagy; cartilage endplate stem cells; exosome; intervertebral disc degeneration.
©2021 The Authors. Stem Cells published by Wiley Periodicals LLC on behalf of AlphaMed Press 2020.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures


Similar articles
-
Senescent cartilage endplate stem cells-derived exosomes induce oxidative stress injury in nucleus pulposus cells and aggravate intervertebral disc degeneration by regulating FOXO3.Free Radic Biol Med. 2025 Jun;233:39-54. doi: 10.1016/j.freeradbiomed.2025.03.027. Epub 2025 Mar 19. Free Radic Biol Med. 2025. PMID: 40118349
-
Exosomes-derived miR-125-5p from cartilage endplate stem cells regulates autophagy and ECM metabolism in nucleus pulposus by targeting SUV38H1.Exp Cell Res. 2022 May 1;414(1):113066. doi: 10.1016/j.yexcr.2022.113066. Epub 2022 Feb 26. Exp Cell Res. 2022. PMID: 35231441
-
Exosomes inhibit ferroptosis to alleviate intervertebral disc degeneration via the p62-KEAP1-NRF2 pathway.Free Radic Biol Med. 2025 May;232:171-184. doi: 10.1016/j.freeradbiomed.2025.02.027. Epub 2025 Feb 20. Free Radic Biol Med. 2025. PMID: 39986487
-
Research Progress on the Role of Cartilage Endplate in Intervertebral Disc Degeneration.Cell Biochem Funct. 2024 Sep;42(7):e4118. doi: 10.1002/cbf.4118. Cell Biochem Funct. 2024. PMID: 39267363 Review.
-
Exosome-mediated Repair of Intervertebral Disc Degeneration: The Potential Role of miRNAs.Curr Stem Cell Res Ther. 2024;19(6):798-808. doi: 10.2174/1574888X18666230504094233. Curr Stem Cell Res Ther. 2024. PMID: 37150986 Review.
Cited by
-
Researches on Stem and Progenitor Cells in Intervertebral Discs: An Analysis of the Scientific Landscape.Stem Cells Int. 2022 Sep 1;2022:1274580. doi: 10.1155/2022/1274580. eCollection 2022. Stem Cells Int. 2022. PMID: 36093440 Free PMC article.
-
Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration.Front Bioeng Biotechnol. 2022 Oct 7;10:1019437. doi: 10.3389/fbioe.2022.1019437. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36277386 Free PMC article. Review.
-
Bioinformatics Analysis Reveals Hub Genes Linked to Programmed Cell Death in Intervertebral Disc Degeneration.Appl Biochem Biotechnol. 2025 Jul;197(7):4475-4493. doi: 10.1007/s12010-025-05243-y. Epub 2025 Apr 30. Appl Biochem Biotechnol. 2025. PMID: 40304990
-
Autophagy activation alleviates annulus fibrosus degeneration via the miR-2355-5p/mTOR pathway.J Orthop Surg Res. 2025 Jan 23;20(1):86. doi: 10.1186/s13018-025-05492-x. J Orthop Surg Res. 2025. PMID: 39849546 Free PMC article.
-
Natural products can modulate inflammation in intervertebral disc degeneration.Front Pharmacol. 2023 Feb 16;14:1150835. doi: 10.3389/fphar.2023.1150835. eCollection 2023. Front Pharmacol. 2023. PMID: 36874009 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

