Microbial production of small peptide: pathway engineering and synthetic biology
- PMID: 33459516
- PMCID: PMC8601181
- DOI: 10.1111/1751-7915.13743
Microbial production of small peptide: pathway engineering and synthetic biology
Abstract
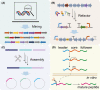
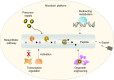
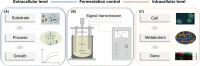
Small peptides are a group of natural products with low molecular weights and complex structures. The diverse structures of small peptides endow them with broad bioactivities and suggest their potential therapeutic use in the medical field. The remaining challenge is methods to address the main limitations, namely (i) the low amount of available small peptides from natural sources, and (ii) complex processes required for traditional chemical synthesis. Therefore, harnessing microbial cells as workhorse appears to be a promising approach to synthesize these bioactive peptides. As an emerging engineering technology, synthetic biology aims to create standard, well-characterized and controllable synthetic systems for the biosynthesis of natural products. In this review, we describe the recent developments in the microbial production of small peptides. More importantly, synthetic biology approaches are considered for the production of small peptides, with an emphasis on chassis cells, the evolution of biosynthetic pathways, strain improvements and fermentation.
© 2021 The Authors. Microbial Biotechnology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Microbial Chassis Development for Natural Product Biosynthesis.Trends Biotechnol. 2020 Jul;38(7):779-796. doi: 10.1016/j.tibtech.2020.01.002. Epub 2020 Feb 3. Trends Biotechnol. 2020. PMID: 32029285 Review.
-
Subcellular compartmentalization in the biosynthesis and engineering of plant natural products.Biotechnol Adv. 2023 Dec;69:108258. doi: 10.1016/j.biotechadv.2023.108258. Epub 2023 Sep 16. Biotechnol Adv. 2023. PMID: 37722606 Review.
-
Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells.Plant Commun. 2021 Aug 9;2(5):100229. doi: 10.1016/j.xplc.2021.100229. eCollection 2021 Sep 13. Plant Commun. 2021. PMID: 34746761 Free PMC article. Review.
-
Microbial production of small medicinal molecules and biologics: From nature to synthetic pathways.Biotechnol Adv. 2018 Dec;36(8):2219-2231. doi: 10.1016/j.biotechadv.2018.10.009. Epub 2018 Oct 29. Biotechnol Adv. 2018. PMID: 30385278 Review.
-
Production of marine-derived bioactive peptide molecules for industrial applications: A reverse engineering approach.Biotechnol Adv. 2024 Dec;77:108449. doi: 10.1016/j.biotechadv.2024.108449. Epub 2024 Sep 10. Biotechnol Adv. 2024. PMID: 39260778 Review.
Cited by
-
Bioactive peptides from food science to pharmaceutical industries: Their mechanism of action, potential role in cancer treatment and available resources.Heliyon. 2024 Nov 22;10(23):e40563. doi: 10.1016/j.heliyon.2024.e40563. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654719 Free PMC article. Review.
-
Peptides Used for Heavy Metal Remediation: A Promising Approach.Int J Mol Sci. 2024 Jun 18;25(12):6717. doi: 10.3390/ijms25126717. Int J Mol Sci. 2024. PMID: 38928423 Free PMC article. Review.
-
Production, Purification, and Potential Health Applications of Edible Seeds' Bioactive Peptides: A Concise Review.Foods. 2021 Nov 4;10(11):2696. doi: 10.3390/foods10112696. Foods. 2021. PMID: 34828976 Free PMC article. Review.
-
Ice Cores as a Source for Antimicrobials: From Bioprospecting to Biodesign.Biodes Res. 2023 Nov 2;5:0024. doi: 10.34133/bdr.0024. eCollection 2023. Biodes Res. 2023. PMID: 37928441 Free PMC article. Review.
-
Lipidated variants of the antimicrobial peptide nisin produced via incorporation of methionine analogs for click chemistry show improved bioactivity.J Biol Chem. 2023 Jul;299(7):104845. doi: 10.1016/j.jbc.2023.104845. Epub 2023 May 19. J Biol Chem. 2023. PMID: 37209826 Free PMC article.
References
-
- Adams, B.L. (2016) The next generation of synthetic biology chassis: moving synthetic biology from the laboratory to the field. ACS Synth Biol 5: 1328–1330. - PubMed
-
- Alexander, D.C. , Rock, J. , He, X. , Brian, P. , Miao, V. , and Baltz, R.H. (2010) Development of a genetic system for combinatorial biosynthesis of lipopeptides in Streptomyces fradiae and heterologous expression of the a54145 biosynthesis gene cluster. Appl Environ Microbiol 76: 6877–6887. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

