Antagonism between germ cell-less and Torso receptor regulates transcriptional quiescence underlying germline/soma distinction
- PMID: 33459591
- PMCID: PMC7843132
- DOI: 10.7554/eLife.54346
Antagonism between germ cell-less and Torso receptor regulates transcriptional quiescence underlying germline/soma distinction
Abstract
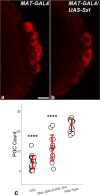
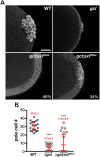
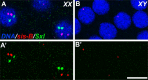
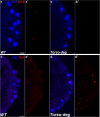
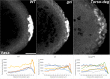
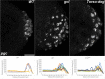
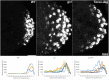
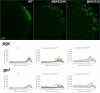
Transcriptional quiescence, an evolutionarily conserved trait, distinguishes the embryonic primordial germ cells (PGCs) from their somatic neighbors. In Drosophila melanogaster, PGCs from embryos maternally compromised for germ cell-less (gcl) misexpress somatic genes, possibly resulting in PGC loss. Recent studies documented a requirement for Gcl during proteolytic degradation of the terminal patterning determinant, Torso receptor. Here we demonstrate that the somatic determinant of female fate, Sex-lethal (Sxl), is a biologically relevant transcriptional target of Gcl. Underscoring the significance of transcriptional silencing mediated by Gcl, ectopic expression of a degradation-resistant form of Torso (torsoDeg) can activate Sxl transcription in PGCs, whereas simultaneous loss of torso-like (tsl) reinstates the quiescent status of gcl PGCs. Intriguingly, like gcl mutants, embryos derived from mothers expressing torsoDeg in the germline display aberrant spreading of pole plasm RNAs, suggesting that mutual antagonism between Gcl and Torso ensures the controlled release of germ-plasm underlying the germline/soma distinction.
Keywords: D. melanogaster; cell fate; developmental biology; germ cell-less; germ cells; germline; torso receptor; transcriptional quiescence.
© 2021, Colonnetta et al.
Conflict of interest statement
MC, LL, LW, GK, EC, PR, PS, DL, GD No competing interests declared
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

