Cannabinoid receptor Type 1 densities reflect social organization in Microtus
- PMID: 33460115
- PMCID: PMC7839313
- DOI: 10.1002/cne.24996
Cannabinoid receptor Type 1 densities reflect social organization in Microtus
Abstract
Across many species, endocannabinoids play an important role in regulating social play, reward, and anxiety. These processes are mediated through at least two distinct cannabinoid receptors (CB), CB1 and CB2. CB1 expression is found in appreciable densities across regions of the brain that integrate memory with socio-spatial information; many of these regions have been directly linked to the neurobiology of pair bonding in monogamous species. Using receptor autoradiography, we provide the first distributional map of CB1 within the brains of closely related monogamous prairie voles and promiscuous meadow voles, and compare receptor densities across sexes and species in limbic regions. We observe CB1-specific signal using [3H] CP-55,940 and [3H] SR141716A, though the latter exhibited a lower signal to noise ratio. We confirmed the presence of CB2 in prairie vole spleen tissue using [3H] CP-55,940. However, we found no evidence of CB2 in the brain using either [3H] CP-55,940 or [3H] A-836,339. The overall distribution of putative CB1 in the brain was similar across vole species and followed the pattern of CB1 expression observed in other species-high intensity binding within the telencephalon, moderate binding within the diencephalon, and mild binding within the mesencephalon and metencephalon (aside from the cerebellar cortex). However, we found profound differences in CB1 densities across species, with prairie voles having higher CB1 binding in regions implicated in social attachment and spatial memory (e.g., periaqueductal gray, hippocampus). These findings suggest that CB1 densities, but not distribution, correlate with the social systems of vole species.
Keywords: CB1; CB2; endocannabinoids; monogamy; pair bonding; social behavioral neural network.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Figures

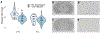

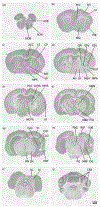

References
-
- Albers HE (2015). Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Frontiers in Neuroendocrinology, 35, 49–71. https://doi.org/http://dx.doi.org/10/1016/j.yfrne.2014.07.001 - PMC - PubMed
-
- Aragona BJ, & Wang ZX (2004). The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. I.L.A.R.Journal, 45(1), 35–45. - PubMed
-
- Boonstra R, Xia X, & Pavone L (1993). Mating system of the meadow vole, Microtus pennsylvanicus. Behavioral Ecology, 4(1), 83–89. 10.1093/beheco/4.1.83 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

