Response to ibudilast treatment according to progressive multiple sclerosis disease phenotype
- PMID: 33460301
- PMCID: PMC7818089
- DOI: 10.1002/acn3.51251
Response to ibudilast treatment according to progressive multiple sclerosis disease phenotype
Abstract
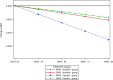
Objective: Determine whether a treatment effect of ibudilast on brain atrophy rate differs between participants with primary (PPMS) and secondary (SPMS) progressive multiple sclerosis.
Background: Progressive forms of MS are both associated with continuous disability progression. Whether PPMS and SPMS differ in treatment response remains unknown.
Design/methods: SPRINT-MS was a randomized, placebo-controlled 96-week phase 2 trial in both PPMS (n = 134) and SPMS (n = 121) patients. The effect of PPMS and SPMS phenotype on the rate of change of brain atrophy measured by brain parenchymal fraction (BPF) was examined by fitting a three-way interaction linear-mixed model. Adjustment for differences in baseline demographics, disease measures, and brain size was explored.
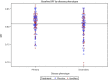
Results: Analysis showed that there was a three-way interaction between the time, treatment effect, and disease phenotype (P < 0.06). After further inspection, the overall treatment effect was primarily driven by patients with PPMS (P < 0.01), and not by patients with SPMS (P = 0.97). This difference may have been due to faster brain atrophy progression seen in the PPMS placebo group compared to SPMS placebo (P < 0.02). Although backward selection (P < 0.05) retained age, T2 lesion volume, RNFL, and longitudinal diffusivity as significant baseline covariates in the linear-mixed model, the adjusted overall treatment effect was still driven by PPMS (P < 0.01).
Interpretation: The previously reported overall treatment effect of ibudilast on worsening of brain atrophy in progressive MS appears to be driven by patients with PPMS that may be, in part, because of the faster atrophy progression rates seen in the placebo-treated group.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors have nothing to report as a relevant conflict of interest to report with respect to this study.
Figures
References
-
- Mahad DH, Trapp BD, Lassmann H. Progressive multiple sclerosis 1 Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015;14:183–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources