SARS-CoV-2 Vaccines: Much Accomplished, Much to Learn
- PMID: 33460347
- PMCID: PMC7839932
- DOI: 10.7326/M21-0111
SARS-CoV-2 Vaccines: Much Accomplished, Much to Learn
Abstract
As COVID vaccines roll out, internists and other health care providers are being turned to as trusted sources of information for patients and communities. Here, experts from NIAID outline the current state of knowledge regarding such vaccines. They contrast vaccine platforms, summarize clinical trial data regarding efficacy and safety, and comment on key questions including the ability of current vaccines to protect against infection and to decrease the prevalence of virus in the community.
Conflict of interest statement
Figures
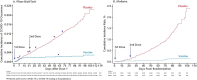
References
-
- Vaccines and Related Biological Products Advisory Committee Meeting, FDA Briefing Document: Pfizer-BioNTech COVID-19 Vaccine. U.S. Food and Drug Adminstration; 10 December 2020. Accessed at www.fda.gov/media/144245/download on 8 January 2021.
-
- Vaccines and Related Biological Products Advisory Committee Meeting, FDA Briefing Document: Moderna COVID-19 Vaccine. U.S. Food and Drug Administration; 17 December 2020. Accessed at www.fda.gov/media/144434/download on 8 January 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
