Strain Energy Decay Predicts Elastic Registration Accuracy From Intraoperative Data Constraints
- PMID: 33460370
- PMCID: PMC8117369
- DOI: 10.1109/TMI.2021.3052523
Strain Energy Decay Predicts Elastic Registration Accuracy From Intraoperative Data Constraints
Abstract
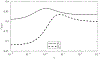
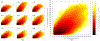
Image-guided intervention for soft tissue organs depends on the accuracy of deformable registration methods to achieve effective results. While registration techniques based on elastic theory are prevalent, no methods yet exist that can prospectively estimate registration uncertainty to regulate sources and mitigate consequences of localization error in deforming organs. This paper introduces registration uncertainty metrics based on dispersion of strain energy from boundary constraints to predict the proportion of target registration error (TRE) remaining after nonrigid elastic registration. These uncertainty metrics depend on the spatial distribution of intraoperative constraints provided to registration with relation to patient-specific organ geometry. Predictive linear and bivariate gamma models are fit and cross-validated using an existing dataset of 6291 simulated registration examples, plus 699 novel simulated registrations withheld for independent validation. Average uncertainty and average proportion of TRE remaining after elastic registration are strongly correlated ( r = 0.78 ), with mean absolute difference in predicted TRE equivalent to 0.9 ± 0.6 mm (cross-validation) and 0.9 ± 0.5 mm (independent validation). Spatial uncertainty maps also permit localized TRE estimates accurate to an equivalent of 3.0 ± 3.1 mm (cross-validation) and 1.6 ± 1.2 mm (independent validation). Additional clinical evaluation of vascular features yields localized TRE estimates accurate to 3.4 ± 3.2 mm. This work formalizes a lower bound for the inherent uncertainty of nonrigid elastic registrations given coverage of intraoperative data constraints, and demonstrates a relation to TRE that can be predictively leveraged to inform data collection and provide a measure of registration confidence for elastic methods.
Figures

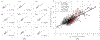

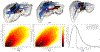

References
-
- Fitzpatrick JM, West JB, and Maurer CR, “Predicting error in rigid-body point-based registration,” IEEE Trans. Med. Imaging, vol. 17, no. 5, pp. 694–702, 1998. - PubMed
-
- Fitzpatrick JM and West JB, “The distribution of target registration error in rigid-body point-based registration,” IEEE Trans. Med. Imaging, vol. 20, no. 9, pp. 917–927, 2001. - PubMed
-
- Wiles AD, Likholyot A, Frantz DD, and Peters TM, “A statistical model for point-based target registration error with anisotropic fiducial localizer error,” IEEE Trans. Med. Imaging, vol. 27, no. 3, pp. 378–390, 2008. - PubMed
-
- Moghari MH and Abolmaesumi P, “Distribution of target registration error for anisotropic and inhomogeneous fiducial localization error,” IEEE Trans. Med. Imaging, vol. 28, no. 6, pp. 799–813, 2009. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

