The Role of Mediobasal Hypothalamic PACAP in the Control of Body Weight and Metabolism
- PMID: 33460433
- PMCID: PMC7875177
- DOI: 10.1210/endocr/bqab012
The Role of Mediobasal Hypothalamic PACAP in the Control of Body Weight and Metabolism
Abstract
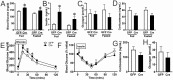
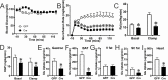
Body energy homeostasis results from balancing energy intake and energy expenditure. Central nervous system administration of pituitary adenylate cyclase activating polypeptide (PACAP) dramatically alters metabolic function, but the physiologic mechanism of this neuropeptide remains poorly defined. PACAP is expressed in the mediobasal hypothalamus (MBH), a brain area essential for energy balance. Ventromedial hypothalamic nucleus (VMN) neurons contain, by far, the largest and most dense population of PACAP in the medial hypothalamus. This region is involved in coordinating the sympathetic nervous system in response to metabolic cues in order to re-establish energy homeostasis. Additionally, the metabolic cue of leptin signaling in the VMN regulates PACAP expression. We hypothesized that PACAP may play a role in the various effector systems of energy homeostasis, and tested its role by using VMN-directed, but MBH encompassing, adeno-associated virus (AAVCre) injections to ablate Adcyap1 (gene coding for PACAP) in mice (Adcyap1MBHKO mice). Adcyap1MBHKO mice rapidly gained body weight and adiposity, becoming hyperinsulinemic and hyperglycemic. Adcyap1MBHKO mice exhibited decreased oxygen consumption (VO2), without changes in activity. These effects appear to be due at least in part to brown adipose tissue (BAT) dysfunction, and we show that PACAP-expressing cells in the MBH can stimulate BAT thermogenesis. While we observed disruption of glucose clearance during hyperinsulinemic/euglycemic clamp studies in obese Adcyap1MBHKO mice, these parameters were normal prior to the onset of obesity. Thus, MBH PACAP plays important roles in the regulation of metabolic rate and energy balance through multiple effector systems on multiple time scales, which highlight the diverse set of functions for PACAP in overall energy homeostasis.
Keywords: Energy balance; Energy expenditure; glucose homeostasis; obesity; thermogenesis; ventromedial hypothalamus.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


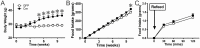


References
-
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087-2102. - PubMed
-
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822-w831. - PubMed
-
- Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12-24. - PubMed
-
- Comerma-Steffensen S, Grann M, Andersen CU, Rungby J, Simonsen U. Cardiovascular effects of current and future anti-obesity drugs. Curr Vasc Pharmacol. 2014;12(3):493-504. - PubMed
-
- Moreira FA, Crippa JA. The psychiatric side-effects of rimonabant. Braz J Psychiatry. 2009;31(2):145-153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK108740/DK/NIDDK NIH HHS/United States
- R01 DK089044/DK/NIDDK NIH HHS/United States
- K08 DK118201/DK/NIDDK NIH HHS/United States
- T32 DK094775/DK/NIDDK NIH HHS/United States
- R01 DK096010/DK/NIDDK NIH HHS/United States
- P50 HD105352/HD/NICHD NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- T32 AA007462/AA/NIAAA NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- T32 HL007374/HL/NHLBI NIH HHS/United States
- R01 DK075632/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK111401/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

