In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana
- PMID: 33460457
- PMCID: PMC8048663
- DOI: 10.1111/nph.17213
In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana
Abstract
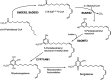
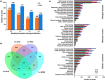
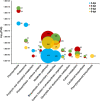
Sorgoleone, a hydrophobic compound exuded from root hair cells of Sorghum spp., accounts for much of the allelopathic activity of the genus. The enzymes involved in the biosynthesis of this compound have been identified and functionally characterized. Here, we report the successful assembly of the biosynthetic pathway and the significant impact of in vivo synthesized sorgoleone on the heterologous host Nicotiana benthamiana. A multigene DNA construct was prepared for the expression of genes required for sorgoleone biosynthesis in planta and deployed in N. benthamiana leaf tissues via Agrobacterium-mediated transient expression. RNA-sequencing was conducted to investigate the effects of sorgoleone, via expression of its biosynthesis pathway, on host gene expression. The production of sorgoleone in agroinfiltrated leaves as detected by gas chromatography/mass spectrometry (GC/MS) resulted in the formation of necrotic lesions, indicating that the compound caused severe phytotoxicity to these tissues. RNA-sequencing profiling revealed significant changes in gene expression in the leaf tissues expressing the pathway during the formation of sorgoleone-induced necrotic lesions. Transcriptome analysis suggested that the compound produced in vivo impaired the photosynthetic system as a result of downregulated gene expression for the photosynthesis apparatus and elevated expression of proteasomal genes which may play a major role in the phytotoxicity of sorgoleone.
Keywords: Nicotiana benthamiana; Sorghum bicolor; Sorgoleone; allelochemical; biosynthetic pathway; differential gene expression.
© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Figures





Similar articles
-
A cytochrome P450 CYP71 enzyme expressed in Sorghum bicolor root hair cells participates in the biosynthesis of the benzoquinone allelochemical sorgoleone.New Phytol. 2018 Apr;218(2):616-629. doi: 10.1111/nph.15037. Epub 2018 Feb 20. New Phytol. 2018. PMID: 29461628 Free PMC article.
-
Alkylresorcinol synthases expressed in Sorghum bicolor root hairs play an essential role in the biosynthesis of the allelopathic benzoquinone sorgoleone.Plant Cell. 2010 Mar;22(3):867-87. doi: 10.1105/tpc.109.072397. Epub 2010 Mar 26. Plant Cell. 2010. PMID: 20348430 Free PMC article.
-
A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs.J Biol Chem. 2008 Feb 8;283(6):3231-3247. doi: 10.1074/jbc.M706587200. Epub 2007 Nov 12. J Biol Chem. 2008. PMID: 17998204
-
An in vivo plant platform to assess genes encoding native and synthetic enzymes for carotenoid biosynthesis.Methods Enzymol. 2022;671:489-509. doi: 10.1016/bs.mie.2022.03.005. Epub 2022 Apr 9. Methods Enzymol. 2022. PMID: 35878991 Review.
-
Sorghum allelopathy--from ecosystem to molecule.J Chem Ecol. 2013 Feb;39(2):142-53. doi: 10.1007/s10886-013-0245-8. Epub 2013 Feb 8. J Chem Ecol. 2013. PMID: 23393005 Review.
Cited by
-
New methods for sorghum transformation in temperate climates.AoB Plants. 2023 Jun 3;15(3):plad030. doi: 10.1093/aobpla/plad030. eCollection 2023 Jun. AoB Plants. 2023. PMID: 37396498 Free PMC article. Review.
-
Enhancing Sorghum Growth: Influence of Arbuscular Mycorrhizal Fungi and Sorgoleone.Microorganisms. 2025 Feb 15;13(2):423. doi: 10.3390/microorganisms13020423. Microorganisms. 2025. PMID: 40005789 Free PMC article.
-
Identification and Functional Characterization of Oxidosqualene Cyclases from Medicinal Plant Hoodia gordonii.Plants (Basel). 2024 Jan 14;13(2):231. doi: 10.3390/plants13020231. Plants (Basel). 2024. PMID: 38256784 Free PMC article.
-
Variation on a theme: the structures and biosynthesis of specialized fatty acid natural products in plants.Plant J. 2022 Aug;111(4):954-965. doi: 10.1111/tpj.15878. Epub 2022 Jul 18. Plant J. 2022. PMID: 35749584 Free PMC article. Review.
-
In planta Female Flower Agroinfiltration Alters the Cannabinoid Composition in Industrial Hemp (Cannabis sativa L.).Front Plant Sci. 2022 Jul 21;13:921970. doi: 10.3389/fpls.2022.921970. eCollection 2022. Front Plant Sci. 2022. PMID: 35941940 Free PMC article.
References
-
- Baerson SR, Dayan FE, Rimando AM, Nanayakkara NP, Liu CJ, Schroder J, Fishbein M, Pan Z, Kagan IA, Pratt LH et al. 2008. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. Journal of Biological Chemistry 283: 3231–3247. - PubMed
-
- Bally J, Jung H, Mortimer C, Naim F, Philips JG, Hellens R, Bombarely A, Goodin MM, Waterhouse PM. 2018. The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annual Review of Phytopathology 56: 405–426. - PubMed
-
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clinical Chemistry 55: 611–622. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

