Involvement of Autophagy in Levodopa-Induced Dyskinesia
- PMID: 33460487
- PMCID: PMC8248404
- DOI: 10.1002/mds.28480
Involvement of Autophagy in Levodopa-Induced Dyskinesia
Abstract
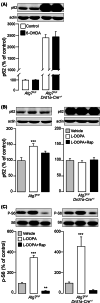
Background: Autophagy is intensively studied in cancer, metabolic and neurodegenerative diseases, but little is known about its role in pathological conditions linked to altered neurotransmission. We examined the involvement of autophagy in levodopa (l-dopa)-induced dyskinesia, a frequent motor complication developed in response to standard dopamine replacement therapy in parkinsonian patients.
Methods: We used mouse and non-human primate models of Parkinson's disease to examine changes in autophagy associated with chronic l-dopa administration and to establish a causative link between impaired autophagy and dyskinesia.
Results: We found that l-dopa-induced dyskinesia is associated with accumulation of the autophagy-specific substrate p62, a marker of autophagy deficiency. Increased p62 was observed in a subset of projection neurons located in the striatum and depended on l-dopa-mediated activation of dopamine D1 receptors, and mammalian target of rapamycin. Inhibition of mammalian target of rapamycin complex 1 with rapamycin counteracted the impairment of autophagy produced by l-dopa, and reduced dyskinesia. The anti-dyskinetic effect of rapamycin was lost when autophagy was constitutively suppressed in D1 receptor-expressing striatal neurons, through inactivation of the autophagy-related gene protein 7.
Conclusions: These findings indicate that augmented responsiveness at D1 receptors leads to dysregulated autophagy, and results in the emergence of l-dopa-induced dyskinesia. They further suggest the enhancement of autophagy as a therapeutic strategy against dyskinesia. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: l-dopa; Parkinson's disease; autophagy; p62; striatum.
© 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Conflict of interest statement
EB is a director and a shareholder of Motac Neuroscience Ltd. The other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

