Past, present and future role of retinal imaging in neurodegenerative disease
- PMID: 33460813
- PMCID: PMC8280255
- DOI: 10.1016/j.preteyeres.2020.100938
Past, present and future role of retinal imaging in neurodegenerative disease
Abstract
Retinal imaging technology is rapidly advancing and can provide ever-increasing amounts of information about the structure, function and molecular composition of retinal tissue in humans in vivo. Most importantly, this information can be obtained rapidly, non-invasively and in many cases using Food and Drug Administration-approved devices that are commercially available. Technologies such as optical coherence tomography have dramatically changed our understanding of retinal disease and in many cases have significantly improved their clinical management. Since the retina is an extension of the brain and shares a common embryological origin with the central nervous system, there has also been intense interest in leveraging the expanding armamentarium of retinal imaging technology to understand, diagnose and monitor neurological diseases. This is particularly appealing because of the high spatial resolution, relatively low-cost and wide availability of retinal imaging modalities such as fundus photography or OCT compared to brain imaging modalities such as magnetic resonance imaging or positron emission tomography. The purpose of this article is to review and synthesize current research about retinal imaging in neurodegenerative disease by providing examples from the literature and elaborating on limitations, challenges and future directions. We begin by providing a general background of the most relevant retinal imaging modalities to ensure that the reader has a foundation on which to understand the clinical studies that are subsequently discussed. We then review the application and results of retinal imaging methodologies to several prevalent neurodegenerative diseases where extensive work has been done including sporadic late onset Alzheimer's Disease, Parkinson's Disease and Huntington's Disease. We also discuss Autosomal Dominant Alzheimer's Disease and cerebrovascular small vessel disease, where the application of retinal imaging holds promise but data is currently scarce. Although cerebrovascular disease is not generally considered a neurodegenerative process, it is both a confounder and contributor to neurodegenerative disease processes that requires more attention. Finally, we discuss ongoing efforts to overcome the limitations in the field and unmet clinical and scientific needs.
Keywords: Alzheimer's disease; Cerebral small vessel disease; Huntington's disease; Imaging; Parkinson's disease; Retina.
Copyright © 2021 Elsevier Ltd. All rights reserved.
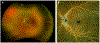
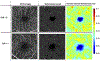
Figures









References
-
- Adam CR, Shrier E, Ding Y, Glazman S, Bodis-Wollner I, 2013. Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J. Neuro Ophthalmol 33 (2), 137–142. - PubMed
-
- Adler DC, Ko TH, Fujimoto JG, 2004. Speckle reduction in optical coherence tomography images by use of a spatially adaptive wavelet filter. Opt. Lett 29 (24), 2878–2880. - PubMed
-
- Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK, Kim JM, Woo SJ, Kim KW, Jeon B, 2018. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 91 (11), e1003–e1012. - PubMed
-
- Alber J, Goldfarb D, Thompson LI, Arthur E, Hernandez K, Cheng D, DeBuc DC, Cordeiro F, Provetti-Cunha L, den Haan J, Van Stavern GP, Salloway SP, Sinoff S, Snyder PJ, 2020. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 16 (1), 229–243. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

