Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells
- PMID: 33460824
- PMCID: PMC8016702
- DOI: 10.1016/j.trsl.2021.01.005
Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells
Abstract
Oligodendrocyte progenitor cells (OPCs) in the infant brain give rise to mature oligodendrocytes that myelinate CNS axons. OPCs are particularly vulnerable to oxidative stress that occurs in many forms of brain injury. One common cause of infant brain injury is neonatal intraventricular hemorrhage (IVH), which releases blood into the CSF and brain parenchyma of preterm infants. Although blood contains the powerful oxidant hemoglobin, the direct effects of hemoglobin on OPCs have not been studied. We utilized a cell culture system to test if hemoglobin induced free radical production and mitochondrial dysfunction in OPCs. We also tested if phenelzine (PLZ), an FDA-approved antioxidant drug, could protect OPCs from hemoglobin-induced oxidative stress. OPCs were isolated from Sprague Dawley rat pups and exposed to hemoglobin with and without PLZ. Outcomes assessed included intracellular reactive oxygen species levels using 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA) fluorescent dye, oxygen consumption using the XFe96 Seahorse assay, and proliferation measured by BrdU incorporation assay. Hemoglobin induced oxidative stress and impaired mitochondrial function in OPCs. PLZ treatment reduced hemoglobin-induced oxidative stress and improved OPC mitochondrial bioenergetics. The effects of hemoglobin and PLZ on OPC proliferation were not statistically significant, but showed trends towards hemoglobin reducing OPC proliferation and PLZ increasing OPC proliferation (P=0.06 for both effects). Collectively, our results indicate that hemoglobin induces mitochondrial dysfunction in OPCs and that antioxidant therapy reduces these effects. Therefore, antioxidant therapy may hold promise for white matter diseases in which hemoglobin plays a role, such as neonatal IVH.
Keywords: Free radicals; Intraventricular hemorrhage; Seahorse; White matter.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Figures


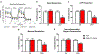



References
-
- Hart AR, Whitby EW, Griffiths PD, et al. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol 2008;50:655–63. - PubMed
-
- Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev 2006;12:129–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

