First-line treatment of metastatic clear cell renal cell carcinoma: a decision-making analysis among experts
- PMID: 33460963
- PMCID: PMC7815472
- DOI: 10.1016/j.esmoop.2020.100030
First-line treatment of metastatic clear cell renal cell carcinoma: a decision-making analysis among experts
Abstract
Background: The treatment landscape of metastatic clear cell renal cell carcinoma (mccRCC) has been transformed by targeted therapies with tyrosine kinase inhibitors (TKI) and more recently by the incorporation of immune checkpoint inhibitors (ICI). Today, a spectrum of single agent TKI to TKI/ICI and ICI/ICI combinations can be considered and the choice of the best regimen is complex.
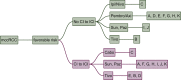
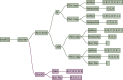
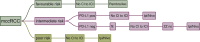
Materials and methods: We performed an updated decision-making analysis among 11 international kidney cancer experts. Each expert provided their treatment strategy and relevant decision criteria in the first line treatment of mccRCC. After the collection of all input a list of unified decision criteria was determined and compatible decision trees were created. We used a methodology based on diagnostic nodes, which allows for an automated cross-comparison of decision trees, to determine the most common treatment recommendations as well as deviations.
Results: Diverse parameters were considered relevant for treatment selection, various drugs and drug combinations were recommended by the experts. The parameters, chosen by the experts, were performance status, International Metastatic renal cell carcinoma Database Consortium (IMDC) risk group, PD-L1 status, zugzwang and contraindication to immunotherapy. The systemic therapies selected for first line treatment were sunitinib, pazopanib, tivozanib, cabozantinib, ipilimumab/nivolumab or pembrolizumab/axitinib.
Conclusion: A wide spectrum of treatment recommendations based on multiple decision criteria was demonstrated. Significant inter-expert variations were observed. This demonstrates how data from randomized trials are implemented differently when transferred into daily practice.
Keywords: clear cell renal cell carcinoma; decision-making; immune checkpoint inhibitor; systemic treatment; tyrosine kinase inhibitor.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure SA: MSD (C/A), Sanofi-Genzyme (C/A) recipient: my institution. MSchmaus: none. TE: AstraZeneca Personal: (RF, E, OI), Bayer (RF), Pfizer (RF), Roche (E, OI); Institution: AstraZeneca (RF), Roche (RF). BE: Pfizer (C/A), BMS (C/A), Ipsen (C/A), Roche (C/A), Oncorena (C/A), Aveo (C/A). VG: Astra Zeneca (CA, H, OI; RF), Bristol-Myers Squibb (C/A, H, OI; RF), Roche Pharma AG (C/A, H), MSD Oncology (C/A, H, OI; RF), Ipsen (C/A, H; RF), Bayer (H; RF), Merck Serono (C/A, H), Janssen Cliag (C/A, H), Pfizer (C/A, H), Lilly (C/A, H), PharmaMar (H), EUSAPharm (C/A, H), Novartis (C/A, H, RF), EISAI (H), Onkowissen (C/A). JL: Achilles Therapeutics (C/A, grant support), Bristol-Myers Squibb (C/A, grant support), Merck Sharp & Dohme (C/A, grant support), Nektar (C/A, grant support), Novartis (C/A, grant support), Pfizer (C/A, grant support), Roche–Genentech (C/A, grant support), Immunocore (C/A, grant support), AstraZeneca (C/A), Boston Biomedical (C/A), Eisai (C/A), EUSA Pharma (C/A), GlaxoSmithKline (C/A), Ipsen (C/A), Imugen (C/A), Incyte (C/A), iOnctura (C/A), Kymab (C/A), Merck Serono (C/A), Pierre Fabre (C/A), Secama (C/A), Vitaccess (C/A), Covance (C/A), Aveo (C/A), Pharmacyclics (C/A). DMcD: BMS (H, C/A), Pfizer (H, C/A), Merck (H, C/A), Alkermes, Inc. (H, C/A). JO: none. CP: Bristol-Myers Squibb (personal fees), Merck Sharpe & Dohme (personal fees), Novartis (personal fees), Ipsen (personal fees), EUSA (personal fees), Eisai (personal fees), Janssen (personal fees), AstraZeneca (personal fees), General Electric (personal fees), Pfizer (grants and personal fees). BIR: Merck (C/A), BMS (C/A), AVEO (C/A), Pfizer (C/A), Roche (C/A), Pfizer (RF), Merck (RF), BMS (RF), AVEO (RF), Astra-Zeneca (RF), Roche (RF). MSchmidinger: Pfizer, BMS, Ipsen, MSD, Merck, Exelixis, EISAI, EUSA, Roche, Novartis, Alkermes. CNS: Pfizer (C/A), MSD (C/A), Merck (C/A), AstraZeneca (C/A), Astellas (C/A), Sanofi-Genzyme (C/A), Roche-Genentech (C/A), Incyte (C/A). CR: Pfizer (C/A), Bristol-Myers Squibb (C/A), Roche Pharma AG (C/A), MSD Oncology (C/A), Merck (Schweiz) AG (C/A) recipient for all: my institution. Astellas Pharma (RF) recipient: my institution. PMP: AstraZeneca (RF), Celgene (RF), Takeda (RF): Educational grants to the institution. Legend: (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Figures

References
-
- Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. - PubMed
-
- Motzer R.J., Mazumdar M., Bacik J., Russo P., Berg W.J., Metz E.M. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18(9):1928–1935. - PubMed
-
- Motzer R.J., Hutson T.E., Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. - PubMed
-
- Sternberg C.N., Davis I.D., Mardiak J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. - PubMed
-
- Hudes G., Carducci M., Tomczak P. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

