Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death
- PMID: 33461384
- PMCID: PMC8632287
- DOI: 10.1080/15548627.2021.1874208
Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death
Abstract
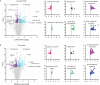
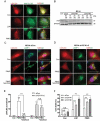
Increasing evidence suggests that induction of lethal macroautophagy/autophagy carries potential significance for the treatment of glioblastoma (GBM). In continuation of previous work, we demonstrate that pimozide and loperamide trigger an ATG5- and ATG7 (autophagy related 5 and 7)-dependent type of cell death that is significantly reduced with cathepsin inhibitors and the lipid reactive oxygen species (ROS) scavenger α-tocopherol in MZ-54 GBM cells. Global proteomic analysis after treatment with both drugs also revealed an increase of proteins related to lipid and cholesterol metabolic processes. These changes were accompanied by a massive accumulation of cholesterol and other lipids in the lysosomal compartment, indicative of impaired lipid transport/degradation. In line with these observations, pimozide and loperamide treatment were associated with a pronounced increase of bioactive sphingolipids including ceramides, glucosylceramides and sphingoid bases measured by targeted lipidomic analysis. Furthermore, pimozide and loperamide inhibited the activity of SMPD1/ASM (sphingomyelin phosphodiesterase 1) and promoted induction of lysosomal membrane permeabilization (LMP), as well as release of CTSB (cathepsin B) into the cytosol in MZ-54 wild-type (WT) cells. Whereas LMP and cell death were significantly attenuated in ATG5 and ATG7 knockout (KO) cells, both events were enhanced by depletion of the lysophagy receptor VCP (valosin containing protein), supporting a pro-survival function of lysophagy under these conditions. Collectively, our data suggest that pimozide and loperamide-driven autophagy and lipotoxicity synergize to induce LMP and cell death. The results also support the notion that simultaneous overactivation of autophagy and induction of LMP represents a promising approach for the treatment of GBM.Abbreviations: ACD: autophagic cell death; AKT1: AKT serine/threonine kinase 1; ATG5: autophagy related 5; ATG7: autophagy related 7; ATG14: autophagy related 14; CERS1: ceramide synthase 1; CTSB: cathepsin B; CYBB/NOX2: cytochrome b-245 beta chain; ER: endoplasmatic reticulum; FBS: fetal bovine serum; GBM: glioblastoma; GO: gene ontology; HTR7/5-HT7: 5-hydroxytryptamine receptor 7; KD: knockdown; KO: knockout; LAMP1: lysosomal associated membrane protein 1; LAP: LC3-associated phagocytosis; LMP: lysosomal membrane permeabilization; MAP1LC3B: microtubule associated protein 1 light chain 3 beta; MTOR: mechanistic target of rapamycin kinase; RB1CC1: RB1 inducible coiled-coil 1; ROS: reactive oxygen species; RPS6: ribosomal protein S6; SMPD1/ASM: sphingomyelin phosphodiesterase 1; VCP/p97: valosin containing protein; WT: wild-type.
Keywords: Acid sphingomyelinase; autophagy-dependent cell death; brain tumors; cholesterol metabolism; drug repurposing; er stress; lysophagy.
Conflict of interest statement
All authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
