Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department
- PMID: 33461884
- PMCID: PMC7552969
- DOI: 10.1016/j.annemergmed.2020.10.008
Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department
Abstract
Study objective: Accurate diagnostic testing to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is critical. Although highly specific, SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) has been shown in clinical practice to be affected by a noninsignificant proportion of false-negative results. This study seeks to explore whether the integration of lung ultrasonography with clinical evaluation is associated with increased sensitivity for the diagnosis of coronavirus disease 2019 pneumonia, and therefore may facilitate the identification of false-negative SARS-CoV-2 RT-PCR results.
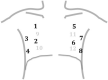
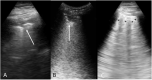
Methods: This prospective cohort study enrolled consecutive adult patients with symptoms potentially related to SARS-CoV-2 infection who were admitted to the emergency department (ED) of an Italian academic hospital. Immediately after the initial assessment, a lung ultrasonographic evaluation was performed and the likelihood of SARS-CoV-2 infection, based on both clinical and lung ultrasonographic findings ("integrated" assessment), was recorded. RT-PCR SARS-CoV-2 detection was subsequently performed.
Results: We enrolled 228 patients; 107 (46.9%) had SARS-CoV-2 infection. Sensitivity and negative predictive value of the clinical-lung ultrasonographic integrated assessment were higher than first RT-PCR result (94.4% [95% confidence interval {CI} 88.2% to 97.9%] versus 80.4% [95% CI 71.6% to 87.4%] and 95% [95% CI 89.5% to 98.2%] versus 85.2% [95% CI 78.3% to 90.6%], respectively). Among the 142 patients who initially had negative RT-PCR results, 21 tested positive at a subsequent molecular test performed within 72 hours. All these false-negative cases were correctly identified by the integrated assessment.
Conclusion: This study suggests that, in patients presenting to the ED with symptoms commonly associated with SARS-CoV-2 infection, the integration of lung ultrasonography with clinical evaluation has high sensitivity and specificity for coronavirus disease 2019 pneumonia and it may help to identify false-negative results occurring with RT-PCR.
Copyright © 2020 American College of Emergency Physicians. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sharfstein J.M., Becker S.J., Mello M.M. Diagnostic testing for the novel coronavirus. JAMA. 2020;323:1437–1438. - PubMed
-
- Marcotte L.M., Liao J.M. Incorporating test characteristics into SARS-CoV-2 testing policy—sense and sensitivity. https://jamanetwork.com/channels/health-forum/fullarticle/2764750 Available at: - PubMed
-
- Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383:e38. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

