Drug reaction with eosinophilia and systemic symptoms syndrome secondary to acetazolamide associated with markedly elevated procalcitonin
- PMID: 33462002
- PMCID: PMC7813420
- DOI: 10.1136/bcr-2020-236966
Drug reaction with eosinophilia and systemic symptoms syndrome secondary to acetazolamide associated with markedly elevated procalcitonin
Abstract
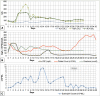
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is an important cause of multi-organ dysfunction and can mimic other disorders including sepsis. We describe a patient presenting with septic shock and accompanying high procalcitonin. Although initially treated empirically with antibiotics, the emergence of eosinophilia during the admission lead to a revised diagnosis of DRESS syndrome, presumed secondary to acetazolamide. This case highlights the importance of regular clinical assessment and re-evaluation is key in identifying emerging features such as eosinophilia, rash and organ dysfunction, which can secure the diagnosis. Furthermore, the case also highlights that acetazolamide may be a rare cause of DRESS syndrome.
Keywords: adult intensive care; immunology; ophthalmology; unwanted effects / adverse reactions.
© BMJ Publishing Group Limited 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
C-Reactive Protein and Procalcitonin in Case Reports of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome.Int Arch Allergy Immunol. 2018;176(1):44-54. doi: 10.1159/000487670. Epub 2018 Apr 13. Int Arch Allergy Immunol. 2018. PMID: 29656292
-
DRESS syndrome presenting with acute hypoxic respiratory failure and delayed eosinophilia.BMJ Case Rep. 2025 May 7;18(5):e262253. doi: 10.1136/bcr-2024-262253. BMJ Case Rep. 2025. PMID: 40341081 Free PMC article.
-
Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome associated with azithromycin presenting like septic shock: a case report.J Med Case Rep. 2014 Oct 8;8:332. doi: 10.1186/1752-1947-8-332. J Med Case Rep. 2014. PMID: 25297051 Free PMC article.
-
Antitubercular therapy causing drug reaction with eosinophilia and systemic symptoms manifesting multi-organ dysfunction syndrome and death in an elderly patient: A case report with review of literature.Int J Mycobacteriol. 2023 Jul-Sep;12(3):360-363. doi: 10.4103/ijmy.ijmy_8_23. Int J Mycobacteriol. 2023. PMID: 37721245 Review.
-
Patch testing in drug reaction with eosinophilia and systemic symptoms (DRESS): A literature review.Contact Dermatitis. 2022 Jun;86(6):443-479. doi: 10.1111/cod.14090. Epub 2022 Mar 29. Contact Dermatitis. 2022. PMID: 35233782 Review.
Cited by
-
Demystifying drug reaction with eosinophilia and systemic symptoms (DRESS): a review of the literature and guidelines for management.Arch Dermatol Res. 2024 Sep 26;316(9):644. doi: 10.1007/s00403-024-03389-z. Arch Dermatol Res. 2024. PMID: 39325061 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
