Bleeding complications of thromboprophylaxis with dabigatran, nadroparin or rivaroxaban for 6 weeks after total knee arthroplasty surgery: a randomised pilot study
- PMID: 33462096
- PMCID: PMC7813324
- DOI: 10.1136/bmjopen-2020-040336
Bleeding complications of thromboprophylaxis with dabigatran, nadroparin or rivaroxaban for 6 weeks after total knee arthroplasty surgery: a randomised pilot study
Abstract
Objectives: For the non-vitamin-K oral anticoagulants, data on bleeding when used for 42 days as thromboprophylaxis after total knee arthroplasty (TKA) are scarce. This pilot study assessed feasibility of a multicentre randomised clinical trial to evaluate major and clinically relevant non-major bleeding during 42-day use of dabigatran, nadroparin and rivaroxaban after TKA.
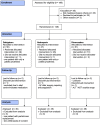
Patients and methods: In 70 weeks, between July 2012 and November 2013, 198 TKA patients were screened for eligibility in the Martini Hospital (Groningen, the Netherlands). Patients were randomly assigned to dabigatran (n=45), nadroparin (n=45) or rivaroxaban (n=48). The primary outcome was the combined endpoint of major bleeding and clinically relevant non-major bleeding. Secondary endpoints of this study were the occurrence of clinical venous thromboembolism (VTE) (pulmonary embolism or deep venous thrombosis), compliance, duration of hospital stay, rehospitalisation, adverse events and Knee Injury and Osteoarthritis Outcome Score (KOOS).
Results: The primary outcome was observed in 33.3% (95% CI 20.0% to 49.0%), 24.4% (95% CI 12.9% to 39.5%) and 27.1% (95% CI 15.3% to 41.8%) of patients who received dabigatran, nadroparin or rivaroxaban, respectively (p=0.67). Major bleeding was found in two patients who received nadroparin (p=0.21). Clinically relevant non-major bleeding was observed in 33.3% (95% CI 20.0% to 49.0%), 22.2% (95% CI 11.2% to 37.1%) and 27.1% (95% CI 15.3% to 41.8%) for dabigatran, nadroparin and rivaroxaban, respectively (p=0.51). Wound haematoma was the most observed bleeding event. VTE was found in one patient who received dabigatran (p=0.65). The presurgery and postsurgery KOOS qQuestionnaires were available for 32 (71%), 35 (77%) and 35 (73%) patients for dabigatran, nadroparin and rivaroxaban, respectively. KOOS was highly variable, and no significant difference between treatment groups in mean improvement was observed.
Conclusions: A multicentre clinical trial may be feasible. However, investments will be substantial. No differences in major and clinically relevant non-major bleeding events were found between dabigatran, nadroparin and rivaroxaban during 42 days after TKA. KOOS may not be suitable to detect functional loss due to bleeding.
Trial registration number: NCT01431456.
Keywords: anticoagulation; bleeding disorders & coagulopathies; knee; thromboembolism.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MvH reports grants from Bayer and personal fees from Boehringer Ingelheim during the conduct of the study. Other authors have nothing to report.
Figures
References
-
- Warwick D, Friedman RJ, Agnelli G, et al. . Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the global orthopaedic registry. J Bone Joint Surg Br 2007;89:799–807. 10.1302/0301-620X.89B6.18844 - DOI - PubMed
-
- Falck-Ytter Y, Francis CW, Johanson NA, et al. . Prevention of VTe in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e278S–325. 10.1378/chest.11-2404 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical