Tissue-resident macrophages regulate lymphatic vessel growth and patterning in the developing heart
- PMID: 33462113
- PMCID: PMC7875498
- DOI: 10.1242/dev.194563
Tissue-resident macrophages regulate lymphatic vessel growth and patterning in the developing heart
Abstract
Macrophages are components of the innate immune system with key roles in tissue inflammation and repair. It is now evident that macrophages also support organogenesis, but few studies have characterized their identity, ontogeny and function during heart development. Here, we show that the distribution and prevalence of resident macrophages in the subepicardial compartment of the developing heart coincides with the emergence of new lymphatics, and that macrophages interact closely with the nascent lymphatic capillaries. Consequently, global macrophage deficiency led to extensive vessel disruption, with mutant hearts exhibiting shortened and mis-patterned lymphatics. The origin of cardiac macrophages was linked to the yolk sac and foetal liver. Moreover, the Cx3cr1+ myeloid lineage was found to play essential functions in the remodelling of the lymphatic endothelium. Mechanistically, macrophage hyaluronan was required for lymphatic sprouting by mediating direct macrophage-lymphatic endothelial cell interactions. Together, these findings reveal insight into the role of macrophages as indispensable mediators of lymphatic growth during the development of the mammalian cardiac vasculature.
Keywords: Cardiac lymphatics; Cell adhesion; Coronaries; Hyaluronan; Macrophages; Vessel growth and patterning.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsP.R.R. is co-founder and equity holder in OxStem Cardio, an Oxford University spin-out that seeks to exploit therapeutic strategies stimulating endogenous repair in cardiovascular regenerative medicine.
Figures

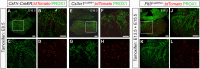


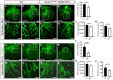
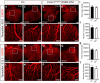
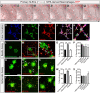
Comment in
-
Cardiac macrophages regulate lymphatic vessel growth during heart development.Nat Rev Cardiol. 2021 Apr;18(4):230. doi: 10.1038/s41569-021-00519-2. Nat Rev Cardiol. 2021. PMID: 33510361 No abstract available.
References
-
- Brachtendorf, G., Kuhn, A., Samulowitz, U., Knorr, R., Gustafsson, E., Potocnik, A. J., Fässler, R. and Vestweber, D. (2001). Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev. Dyn. 222, 410-419. 10.1002/dvdy.1199 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 107457/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- RG/18/5/33532/BHF_/British Heart Foundation/United Kingdom
- RM/13/3/30159/BHF_/British Heart Foundation/United Kingdom
- PG/10/60/28496/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00016/5/MRC_/Medical Research Council/United Kingdom
- MC_UU_00008/2/MRC_/Medical Research Council/United Kingdom
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- RG/08/003/25264/BHF_/British Heart Foundation/United Kingdom
- G0801073/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/5/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_EX_MR/N50192X/1/MRC_/Medical Research Council/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/T017074/1/MRC_/Medical Research Council/United Kingdom
- 091911/B/10/Z/WT_/Wellcome Trust/United Kingdom
- FS/19/31/34158/BHF_/British Heart Foundation/United Kingdom
- WTISSF121302/WT_/Wellcome Trust/United Kingdom
- G0501838/MRC_/Medical Research Council/United Kingdom
- MR/M024962/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

