Omega-3 fatty acids accelerate fledging in an avian marine predator: a potential role of cognition
- PMID: 33462136
- PMCID: PMC7929930
- DOI: 10.1242/jeb.235929
Omega-3 fatty acids accelerate fledging in an avian marine predator: a potential role of cognition
Abstract
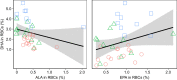
Consuming omega-3 fatty acids (n-3 LCPUFAs) during development improves cognition in mammals, but the effect remains untested in other taxa. In aquatic ecosystems, n-3 LCPUFAs are produced by phytoplankton and bioaccumulate in the food web. Alarmingly, the warming and acidification of aquatic systems caused by climate change impair n-3 LCPUFA production, with an anticipated decrease of 80% by the year 2100. We tested whether n-3 LCPUFA consumption affects the physiology, morphology, behaviour and cognition of the chicks of a top marine predator, the ring-billed gull. Using a colony with little access to n-3 LCPUFAs, we supplemented siblings from 22 fenced nests with contrasting treatments from hatching until fledging; one sibling received n-3 LCPUFA-rich fish oil and the other, a control sucrose solution without n-3 LCPUFAs. Halfway through the nestling period, half the chicks receiving fish oil were switched to the sucrose solution to test whether n-3 LCPUFA intake remains crucial past the main growth phase (chronic versus transient treatments). Upon fledging, n-3 LCPUFAs were elevated in the blood and brains of chicks receiving the chronic treatment, but were comparable to control levels among those receiving the transient treatment. Across the entire sample, chicks with elevated n-3 LCPUFAs in their tissues fledged earlier despite their morphology and activity levels being unrelated to fledging age. Fledging required chicks to escape fences encircling their nest. We therefore interpret fledging age as a possible indicator of cognition, with chicks with improved cognition fledging earlier. These results provide insight into whether declining dietary n-3 LCPUFAs will compromise top predators' problem-solving skills, and thus their ability to survive in a rapidly changing world.
Keywords: Aquatic ecosystem; Bird; Brain development; Docosahexaenoic acid; Eicosapentaenoic acid; Essential fatty acid.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Aponte, V., Locke, S. A., Gentes, M.-L., Giroux, J.-F., Marcogliese, D. J., McLaughlin, D. and Verreault, J. (2014). Effect of habitat use and diet on the gastrointestinal parasite community of an avian omnivore from an urbanized environment. Can. J. Zool. 92, 629-636. 10.1139/cjz-2013-0268 - DOI
-
- Arts, G. H. (2002). Deterioration of Atlantic soft water macrophyte communities by acidification, eutrophication and alkalinisation. Aquat. Bot. 73, 373-393. 10.1016/S0304-3770(02)00031-1 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

