Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer
- PMID: 33462141
- PMCID: PMC7813395
- DOI: 10.1136/jitc-2020-001895
Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer
Erratum in
-
Correction: Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer.J Immunother Cancer. 2021 Oct;9(10):e001895corr1. doi: 10.1136/jitc-2020-001895corr1. J Immunother Cancer. 2021. PMID: 34645677 Free PMC article. No abstract available.
Abstract
Background: Immune checkpoint inhibitors (ICIs), including anti-PD-1 therapy, have limited efficacy in patients with microsatellite stable (MSS) colorectal cancer (CRC). Interleukin 17A (IL-17A) activity leads to a protumor microenvironment, dependent on its ability to induce the production of inflammatory mediators, mobilize myeloid cells and reshape the tumor environment. In the present study, we aimed to investigate the role of IL-17A in resistance to antitumor immunity and to explore the feasibility of anti-IL-17A combined with anti-PD-1 therapy in MSS CRC murine models.
Methods: The expression of programmed cell death-ligand 1 (PD-L1) and its regulation by miR-15b-5p were investigated in MSS CRC cell lines and tissues. The effects of miR-15b-5p on tumorigenesis and anti-PD-1 treatment sensitivity were verified both in vitro and in colitis-associated cancer (CAC) and APCmin/+ murine models. In vivo efficacy and mechanistic studies were conducted using antibodies targeting IL-17A and PD-1 in mice bearing subcutaneous CT26 and MC38 tumors.
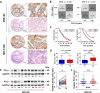
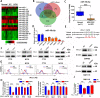
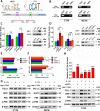
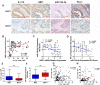
Results: Evaluation of clinical pathological specimens confirmed that PD-L1 mRNA levels are associated with CD8+ T cell infiltration and better prognosis. miR-15b-5p was found to downregulate the expression of PD-L1 at the protein level, inhibit tumorigenesis and enhance anti-PD-1 sensitivity in CAC and APCmin/+ CRC models. IL-17A led to high PD-L1 expression in CRC cells through regulating the P65/NRF1/miR-15b-5p axis. Combined IL-17A and PD-1 blockade had efficacy in CT26 and MC38 tumors, with more cytotoxic T lymphocytes cells and fewer myeloid-derived suppressor cells in tumors.
Conclusions: IL-17A increases PD-L1 expression through the p65/NRF1/miR-15b-5p axis and promotes resistance to anti-PD-1 therapy. Blocking IL-17A improved the efficacy of anti-PD-1 therapy in MSS CRC murine models. IL-17A might serve as a therapeutic target to sensitize patients with MSS CRC to ICI therapy.
Keywords: combination; cytokines; drug therapy; gastrointestinal neoplasms; immunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Overman MJ, McDermott R, Leach JL, et al. . Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
