Plasma membrane integrity in health and disease: significance and therapeutic potential
- PMID: 33462191
- PMCID: PMC7813858
- DOI: 10.1038/s41421-020-00233-2
Plasma membrane integrity in health and disease: significance and therapeutic potential
Abstract
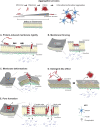
Maintenance of plasma membrane integrity is essential for normal cell viability and function. Thus, robust membrane repair mechanisms have evolved to counteract the eminent threat of a torn plasma membrane. Different repair mechanisms and the bio-physical parameters required for efficient repair are now emerging from different research groups. However, less is known about when these mechanisms come into play. This review focuses on the existence of membrane disruptions and repair mechanisms in both physiological and pathological conditions, and across multiple cell types, albeit to different degrees. Fundamentally, irrespective of the source of membrane disruption, aberrant calcium influx is the common stimulus that activates the membrane repair response. Inadequate repair responses can tip the balance between physiology and pathology, highlighting the significance of plasma membrane integrity. For example, an over-activated repair response can promote cancer invasion, while the inability to efficiently repair membrane can drive neurodegeneration and muscular dystrophies. The interdisciplinary view explored here emphasises the widespread potential of targeting plasma membrane repair mechanisms for therapeutic purposes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

