The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans
- PMID: 33462432
- PMCID: PMC7812346
- DOI: 10.1038/s41573-020-00093-1
The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans
Erratum in
-
Author Correction: The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans.Nat Rev Drug Discov. 2021 Mar;20(3):244. doi: 10.1038/s41573-021-00160-1. Nat Rev Drug Discov. 2021. PMID: 33558696 Free PMC article. No abstract available.
Abstract
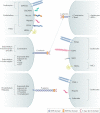
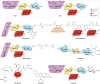
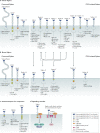
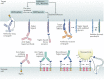
Carbohydrates - namely glycans - decorate every cell in the human body and most secreted proteins. Advances in genomics, glycoproteomics and tools from chemical biology have made glycobiology more tractable and understandable. Dysregulated glycosylation plays a major role in disease processes from immune evasion to cognition, sparking research that aims to target glycans for therapeutic benefit. The field is now poised for a boom in drug development. As a harbinger of this activity, glycobiology has already produced several drugs that have improved human health or are currently being translated to the clinic. Focusing on three areas - selectins, Siglecs and glycan-targeted antibodies - this Review aims to tell the stories behind therapies inspired by glycans and to outline how the lessons learned from these approaches are paving the way for future glycobiology-focused therapeutics.
Conflict of interest statement
C.R.B. is a co-founder of Redwood Bioscience, Enable Biosciences, Palleon Pharmaceuticals, InterVenn Bio, Lycia Therapeutics and OliLux Biosciences, and a member of the Board of Directors of Eli Lilly. B.A.H.S. is a shareholder of GlycoMimetics.
Figures

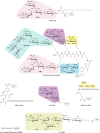
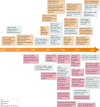
References
-
- Martinez-Palomo A, Braislovsky C, Bernhard W. Ultrastructural modifications of the cell surface and intercellular contacts of some transformed cell strains. Cancer Res. 1969;29:925–937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

