Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis
- PMID: 33462434
- PMCID: PMC7914224
- DOI: 10.1038/s41564-020-00837-0
Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis
Abstract
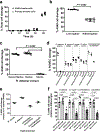
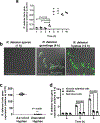
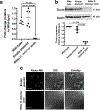
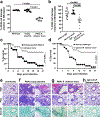
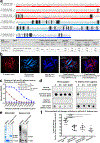
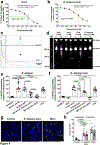
Fungi of the order Mucorales cause mucormycosis, a lethal infection with an incompletely understood pathogenesis. We demonstrate that Mucorales fungi produce a toxin, which plays a central role in virulence. Polyclonal antibodies against this toxin inhibit its ability to damage human cells in vitro and prevent hypovolemic shock, organ necrosis and death in mice with mucormycosis. Inhibition of the toxin in Rhizopus delemar through RNA interference compromises the ability of the fungus to damage host cells and attenuates virulence in mice. This 17 kDa toxin has structural and functional features of the plant toxin ricin, including the ability to inhibit protein synthesis through its N-glycosylase activity, the existence of a motif that mediates vascular leak and a lectin sequence. Antibodies against the toxin inhibit R. delemar- or toxin-mediated vascular permeability in vitro and cross react with ricin. A monoclonal anti-ricin B chain antibody binds to the toxin and also inhibits its ability to cause vascular permeability. Therefore, we propose the name 'mucoricin' for this toxin. Not only is mucoricin important in the pathogenesis of mucormycosis but our data suggest that a ricin-like toxin is produced by organisms beyond the plant and bacterial kingdoms. Importantly, mucoricin should be a promising therapeutic target.
Figures

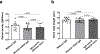




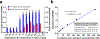

References
-
- Gleissner B, Schilling A, Anagnostopolous I, Siehl I & Thiel E Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma 45, 1351–1360 (2004). - PubMed
-
- Kauffman CA Zygomycosis: reemergence of an old pathogen. Clin Infect Dis 39, 588–590 (2004). - PubMed
-
- Kontoyiannis DP, Wessel VC, Bodey GP & Rolston KV Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 30, 851–856 (2000). - PubMed
-
- Marr KA, Carter RA, Crippa F, Wald A & Corey L Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 34, 909–917 (2002). - PubMed
-
- Siwek GT et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis 39, 584–587 (2004). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

