High-Yield Preparation of Outer Membrane Protein Efflux Pumps by in Vitro Refolding is Concentration Dependent
- PMID: 33462665
- PMCID: PMC8101038
- DOI: 10.1007/s00232-020-00161-y
High-Yield Preparation of Outer Membrane Protein Efflux Pumps by in Vitro Refolding is Concentration Dependent
Abstract
Overexpression of tripartite efflux pump systems in gram-negative bacteria is a principal component of antibiotic resistance. High-yield purification of the outer membrane component of these systems will enable biochemical and structural interrogation of their mechanisms of action and allow testing of compounds that target them. However, preparation of these proteins is typically hampered by low yields, requiring laborious large-scale efforts. If refolding conditions can be found, refolding these proteins from inclusion bodies can lead to increased yields as compared to membrane isolations. A classical method for refolding outer membrane proteins involves unfolding inclusion bodies in urea followed by refolding in lipid or detergent micelles. However, that method has not yet been successful in refolding tripartite efflux pump TolC. Here, we find that refolding TolC from inclusion bodies requires an additional oligomerization enhancing step of sample concentration. We show that by our method of refolding, homotrimeric TolC remains folded in SDS-PAGE, retains binding to an endogenous ligand, and recapitulates the known crystal structure by single particle cryoEM analysis. We find that TolC refolding is concentration dependent. We then extended our method to refolding CmeC, a homologous protein from Campylobacter jejuni, and find that concentration-dependent oligomerization is a general feature of these systems. Because outer membrane efflux pump components are ubiquitous across gram-negative species, we anticipate that incorporating a concentration step in refolding protocols will promote correct refolding allowing for reliable, high-yield preparation of this family of proteins.
Keywords: Antibiotic resistance; In vitro folding; Outer membrane protein.
Figures

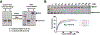
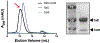

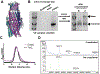

References
-
- Bannwarth M and Schulz GE (2003). “The expression of outer membrane proteins for crystallization.” Biochim Biophys Acta 1610(1): 37–45. - PubMed
-
- Buchanan SK (1999). “Beta-barrel proteins from bacterial outer membranes: structure, function and refolding.” Curr Opin Struct Biol 9(4): 455–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

