IMpower132: Atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients
- PMID: 33462883
- PMCID: PMC8019191
- DOI: 10.1111/cas.14817
IMpower132: Atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients
Abstract
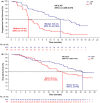
IMpower132 explored the safety and efficacy of atezolizumab plus pemetrexed and platinum-based chemotherapy as first-line treatment for advanced non-small-cell lung cancer (NSCLC). Key eligibility criteria for the phase 3, open-label, IMpower132 study included age ≥18 y, histologically or cytologically confirmed advanced non-squamous NSCLC per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, Eastern Cooperative Oncology Group performance status of 0/1, and no prior systemic treatment for stage IV NSCLC. Patients received atezolizumab (1200 mg) plus pemetrexed (500 mg/m2 ) and cisplatin (75 mg/m2 ) or carboplatin (area under the concentration curve, 6 mg/mL/min) (APP arm) or chemotherapy alone (PP arm). The co-primary study endpoints were overall survival (OS) and investigator-assessed progression-free survival (PFS) per RECIST 1.1 in the intention-to-treat population. A subgroup analysis was conducted in Japanese patients. In the Japanese subgroup (n = 101), median OS was 30.8 (95% CI, 24.3 to not estimable) mo in the APP arm (n = 48) and 22.2 (95% CI, 15.7-30.8) mo in the PP arm (n = 53; hazard ratio [HR], 0.63 [95% CI, 0.36-1.14]). PFS was 12.8 (95% CI, 8.6-16.6) mo in the APP arm vs 4.5 (95% CI, 4.1-6.7) mo in the PP arm (HR, 0.33 [95% CI, 0.21-0.58]). Grade 3/4 treatment-related adverse events (TRAEs) occurred in 68.8% of APP arm patients and 44.2% of PP arm patients. Consistent with global study results, atezolizumab plus pemetrexed and platinum-based chemotherapy improved efficacy and was well tolerated in Japanese patients with advanced NSCLC despite a higher incidence of grade 3/4 TRAEs.
Keywords: IMpower132; Japan; atezolizumab; checkpoint inhibitors; programmed death-ligand 1.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
All authors disclose medical writing support funded by Chugai Pharmaceutical Co., Ltd. Dr. Nishio reports research funds from Ono Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical Co. Ltd., AstraZeneca, MSD, Novartis, Merck Biopharma, Daiichi Sankyo, Boehringer Ingelheim and Takeda Pharmaceutical Co. Ltd.; lecture fees or honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical Co. Ltd., Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Merck Biopharma, Daiichi Sankyo, Takeda Pharmaceutical Co. Ltd., Teijin Pharma and AbbVie. Dr. Saito reports research funds from AstraZeneca, Chugai Pharmaceutical Co. Ltd., and MSD. Dr. Goto reports research funds and lecture fees or honoraria from Chugai Pharmaceutical Co. Ltd. Dr. Watanabe reports lecture fees or honoraria from AstraZeneca and Chugai Pharmaceutical Co. Ltd. Dr. Sueoka‐Aragane reports research funds from AstraZeneca, Boehringer Ingelheim, Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Eli Lilly. Dr. Okuma reports research grants from Chugai Pharmaceutical Co. Ltd., and AbbVie. Dr. Kasahara reports research grants from Boehringer Ingelheim. Dr. Chikamori reports research funds from Chugai Pharmaceutical Co. Ltd, MSD, Bristol Myers Squibb, and Takeda Pharmaceutical Co. Ltd. Dr. Nakagawa is an employee of Chugai Pharmaceutical Co., Ltd. Dr. Kawakami is an employee of Chugai Pharmaceutical Co., Ltd.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- IARC . Globocan Worldwide Fact Sheet 2018. Lyon, France: International Agency for Research on Cancer/World Health Organization; 2018.
-
- Planchard D, Popat S, Kerr K, et al. Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29:iv192‐iv237. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology: Non‐Small Cell Lung Cancer. Plymouth Meeting: National Comprehensive Cancer Network; 2020;v3.0.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

