Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial
- PMID: 33463067
- PMCID: PMC7805402
- DOI: 10.1002/ctm2.265
Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial
Abstract
Background: Osteogenesis imperfecta (OI) is a rare genetic disease characterized by bone fragility, with a wide range in the severity of clinical manifestations. The majority of cases are due to mutations in the COL1A1 or COL1A2 genes, which encode type I collagen. Mesenchymal stem cells (MSCs), as the progenitors of the osteoblasts, the main type I collagen secreting cell type in the bone, have been proposed and tested as an innovative therapy for OI with promising but transient outcomes.
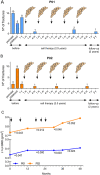
Methods: To overcome the short-term effect of MSCs therapy, we performed a phase I clinical trial based on reiterative infusions of histocompatible MSCs, administered in a 2.5-year period, in two pediatric patients affected by severe and moderate OI. The aim of this study was to assess the safety and effectiveness of this cell therapy in nonimmunosuppressed OI patients. The host response to MSCs was studied by analyzing the sera from OI patients, collected before, during, and after the cell therapy.
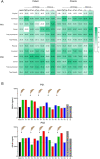
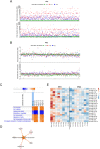
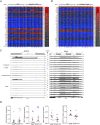
Results: We first demonstrated that the sequential administration of MSCs was safe and improved the bone parameters and quality of life of OI patients along the cell treatment plus 2-year follow-up period. Moreover, the study of the mechanism of action indicated that MSCs therapy elicited a pro-osteogenic paracrine response in patients, especially noticeable in the patient affected by severe OI.
Conclusions: Our results demonstrate the feasibility and potential of reiterative MSCs infusion for two pediatric OI and highlight the paracrine response shown by patients as a consequence of MSCs treatment.
Keywords: cell therapy; mesenchymal stem cell; paracrine mechanism of action; regenerative medicine.
© 2021 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Trophic effects of multiple administration of mesenchymal stem cells in children with osteogenesis imperfecta.Clin Transl Med. 2021 Apr;11(4):e385. doi: 10.1002/ctm2.385. Clin Transl Med. 2021. PMID: 33931966 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
