Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-mesenchymal Transition in Lung Cell Culture Model
- PMID: 33463102
- PMCID: PMC8366022
- DOI: 10.18502/ijaai.v19i4.4110
Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-mesenchymal Transition in Lung Cell Culture Model
Abstract
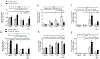
Impaired lung epithelial cell regeneration following injury may contribute to the development of pulmonary fibrosis. Epithelial-mesenchymal transition (EMT) is a critical event in embryonic development, wound healing following injury, and even cancer progression. Previous studies have shown that the combination of transforming growth factor beta-1 (TGFβ1) and fibroblast growth factor 2 (FGF2) induces EMT during cancer metastasis. However, this synergy remains to be elucidated in inducing EMT associated with wound healing after injury. We set out this study to determine the effect of fibroblast growth factor 2 (FGF2) on TGFβ1-induced EMT in the human lung epithelium. BEAS-2B and A549 cells were treated with TGFβ1, FGF2, or both. EMT phenotype was investigated morphologically and by measuring mRNA expression levels; using quantitative real-time PCR. E-cadherin expression was assayed by western blot and immunofluorescence staining. Cell migration was confirmed using a wound-healing assay. TGFβ1 induced a morphological change and a significant increase in cell migration of BEAS-2B cells. TGFβ1 significantly reduced E-cadherin (CDH1) mRNA expression and markedly induced expression of N-cadherin (CDH2), tenascin C (TNC), fibronectin (FN), actin alpha 2 (ACTA2), and collagen I (COL1A1). While FGF2 alone did not significantly alter EMT gene expression, it enhanced TGFβ1-induced suppression of CDH1 and upregulation of ACTA2, but not TNC, FN, and CDH2. FGF2 significantly inhibited TGFβ1-induced COL1A1 expression. Furthermore, FGF2 maintained TGFβ1-induced morphologic changes and increased the migration of TGFβ1-treated cells. This study suggests a synergistic effect between TGFβ1 and FGF2 in inducing EMT in lung epithelial cells, which may play an important role in wound healing and tissue repair after injury.
Keywords: Epithelial cells; Epithelial-mesenchymal transition; Fibroblast growth factor 2; Lung injury; Transforming growth factor beta1.
Conflict of interest statement
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

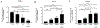


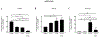



References
-
- Desai O, Winkler J, Minasyan M, Herzog EL. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front Med [Internet]. 2018March20 [cited 2019 Aug 27];5:43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29616220 - PMC - PubMed
-
- King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet [Internet]. 2011December3 [cited 2019 Aug 4];378(9807):1949–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21719092 - PubMed
-
- Li M, Luan F, Zhao Y, Hao H, Zhou Y, Han W, et al. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp Biol Med (Maywood) [Internet]. 2016January [cited 2018 Mar 9];241(1):1–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26361988 - PMC - PubMed
-
- Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology [Internet]. Vol. 19, Respiratory Research. 2018. [cited 2019 Sep 24]. p. 136. Available from: 10.1186/s12931-018-0834-8 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
