Mixed Composition Microribbon Hydrogels Induce Rapid and Synergistic Cartilage Regeneration by Mesenchymal Stem Cells in 3D via Paracrine Signaling Exchange
- PMID: 33463346
- PMCID: PMC10154175
- DOI: 10.1021/acsbiomaterials.0c00131
Mixed Composition Microribbon Hydrogels Induce Rapid and Synergistic Cartilage Regeneration by Mesenchymal Stem Cells in 3D via Paracrine Signaling Exchange
Abstract
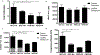
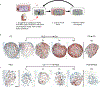
Hydrogels are widely used matrices for mesenchymal stem cell (MSC)-based cartilage regeneration but often result in slow cartilage deposition with inferior mechanical strength. We recently reported a gelatin-based microribbon (μRB) scaffold, which contains macroporosity and substantially enhances the speed of cartilage formation by MSCs in 3D. However, our previous method cannot be used to fabricate different polymers into μRBs, and the effects of varying μRB compositions on MSC cartilage regeneration in 3D remain unknown. Here, we report a method that allows fabricating different polymers [gelatin, chondroitin sulfate, hyaluronic acid, and polyethylene glycol (PEG)] into μRB structures, which can be mixed in any ratio and cross-linked into 3D scaffolds in a modular manner. Mixing glycosaminoglycan μRBs with gelatin or PEG μRBs induced great synergy, resulting in fast cartilage deposition. After only 3 weeks of culture, leading mixed μRB composition reached high compressive strength on par with native cartilage. Such synergy can be recapitulated via exchange of soluble factors secreted by MSCs seeded in different μRB compositions in a dose-dependent manner. Tuning the ratio of mixed μRB compositions allowed further optimization of the quantity and speed of cartilage regeneration by MSCs. Together, our results validate mixed μRB compositions as a novel biomaterial tool for inducing synergy and accelerating MSC-based cartilage regeneration with biomimetic mechanical properties through paracrine signal exchange.
Keywords: cartilage; hydrogels; scaffolds; stem cells; tissue engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Gelatin-Based Microribbon Hydrogels Support Robust MSC Osteogenesis across a Broad Range of Stiffness.ACS Biomater Sci Eng. 2020 Jun 8;6(6):3454-3463. doi: 10.1021/acsbiomaterials.9b01792. Epub 2020 May 27. ACS Biomater Sci Eng. 2020. PMID: 33463171 Free PMC article.
-
Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions.Tissue Eng Part A. 2018 Nov;24(21-22):1631-1640. doi: 10.1089/ten.TEA.2018.0011. Tissue Eng Part A. 2018. PMID: 29926770 Free PMC article.
-
Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo.Theranostics. 2020 May 15;10(13):6035-6047. doi: 10.7150/thno.41096. eCollection 2020. Theranostics. 2020. PMID: 32483436 Free PMC article.
-
Biomimetic hydrogels designed for cartilage tissue engineering.Biomater Sci. 2021 Jun 15;9(12):4246-4259. doi: 10.1039/d0bm01852j. Biomater Sci. 2021. PMID: 33710205 Review.
-
Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids.Polymers (Basel). 2017 Dec 4;9(12):671. doi: 10.3390/polym9120671. Polymers (Basel). 2017. PMID: 30965974 Free PMC article. Review.
Cited by
-
How the mechanical microenvironment of stem cell growth affects their differentiation: a review.Stem Cell Res Ther. 2022 Aug 13;13(1):415. doi: 10.1186/s13287-022-03070-0. Stem Cell Res Ther. 2022. PMID: 35964140 Free PMC article. Review.
-
Recent Advances in Macroporous Hydrogels for Cell Behavior and Tissue Engineering.Gels. 2022 Sep 21;8(10):606. doi: 10.3390/gels8100606. Gels. 2022. PMID: 36286107 Free PMC article. Review.
-
Modulating immune-stem cell crosstalk enables robust bone regeneration via tuning compositions of macroporous scaffolds.NPJ Regen Med. 2025 Jul 1;10(1):33. doi: 10.1038/s41536-025-00421-2. NPJ Regen Med. 2025. PMID: 40595820 Free PMC article.
References
-
- Tan H; Marra KG Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767.
-
- Visser J; Gawlitta D; Benders KEM; Toma SMH; Pouran B; van Weeren PR; Dhert WJA; Malda J Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
