Isolevuglandin-Modified Cardiac Proteins Drive CD4+ T-Cell Activation in the Heart and Promote Cardiac Dysfunction
- PMID: 33463362
- PMCID: PMC7987774
- DOI: 10.1161/CIRCULATIONAHA.120.051889
Isolevuglandin-Modified Cardiac Proteins Drive CD4+ T-Cell Activation in the Heart and Promote Cardiac Dysfunction
Erratum in
-
Correction to: Isolevuglandin-Modified Cardiac Proteins Drive CD4+ T Cell Activation in the Heart and Promote Cardiac Dysfunction.Circulation. 2021 Mar 23;143(12):e794. doi: 10.1161/CIR.0000000000000975. Epub 2021 Mar 22. Circulation. 2021. PMID: 33750212 No abstract available.
Abstract
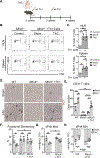
Background: Despite the well-established association between T-cell-mediated inflammation and nonischemic heart failure, the specific mechanisms triggering T-cell activation during the progression of heart failure and the antigens involved are poorly understood. We hypothesized that myocardial oxidative stress induces the formation of isolevuglandin (IsoLG)-modified proteins that function as cardiac neoantigens to elicit CD4+ T-cell receptor (TCR) activation and promote heart failure.
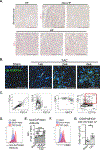
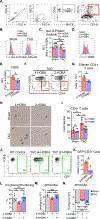
Methods: We used transverse aortic constriction in mice to trigger myocardial oxidative stress and T-cell infiltration. We profiled the TCR repertoire by mRNA sequencing of intramyocardial activated CD4+ T cells in Nur77GFP reporter mice, which transiently express GFP on TCR engagement. We assessed the role of antigen presentation and TCR specificity in the development of cardiac dysfunction using antigen presentation-deficient MhcII-/- mice and TCR transgenic OTII mice that lack specificity for endogenous antigens. We detected IsoLG protein adducts in failing human hearts. We also evaluated the role of reactive oxygen species and IsoLGs in eliciting T-cell immune responses in vivo by treating mice with the antioxidant TEMPOL and the IsoLG scavenger 2-hydroxybenzylamine during transverse aortic constriction, and ex vivo in mechanistic studies of CD4+ T-cell proliferation in response to IsoLG-modified cardiac proteins.
Results: We discovered that TCR antigen recognition increases in the left ventricle as cardiac dysfunction progresses and identified a limited repertoire of activated CD4+ T-cell clonotypes in the left ventricle. Antigen presentation of endogenous antigens was required to develop cardiac dysfunction because MhcII-/- mice reconstituted with CD4+ T cells and OTII mice immunized with their cognate antigen were protected from transverse aortic constriction-induced cardiac dysfunction despite the presence of left ventricle-infiltrated CD4+ T cells. Scavenging IsoLGs with 2-hydroxybenzylamine reduced TCR activation and prevented cardiac dysfunction. Mechanistically, cardiac pressure overload resulted in reactive oxygen species-dependent dendritic cell accumulation of IsoLG protein adducts, which induced robust CD4+ T-cell proliferation.
Conclusions: Our study demonstrates an important role of reactive oxygen species-induced formation of IsoLG-modified cardiac neoantigens that lead to TCR-dependent CD4+ T-cell activation within the heart.
Keywords: heart failure; inflammation; isolevuglandin; oxidative stress.
Conflict of interest statement
Conflict of Interest Disclosures
None
Figures



Similar articles
-
Isolevuglandin scavenger attenuates pressure overload-induced cardiac oxidative stress, cardiac hypertrophy, heart failure and lung remodeling.Free Radic Biol Med. 2019 Sep;141:291-298. doi: 10.1016/j.freeradbiomed.2019.06.029. Epub 2019 Jun 26. Free Radic Biol Med. 2019. PMID: 31254620
-
Targeting of reactive isolevuglandins in mitochondrial dysfunction and inflammation.Redox Biol. 2019 Sep;26:101300. doi: 10.1016/j.redox.2019.101300. Epub 2019 Aug 14. Redox Biol. 2019. PMID: 31437812 Free PMC article.
-
Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation.Cardiovasc Res. 2021 Apr 23;117(5):1358-1371. doi: 10.1093/cvr/cvaa207. Cardiovasc Res. 2021. PMID: 33038226 Free PMC article.
-
Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases.Free Radic Biol Med. 2021 Jan;162:266-273. doi: 10.1016/j.freeradbiomed.2020.10.024. Epub 2020 Oct 21. Free Radic Biol Med. 2021. PMID: 33099003 Review.
-
Mechanisms of isolevuglandin-protein adduct formation in inflammation and hypertension.Prostaglandins Other Lipid Mediat. 2018 Nov;139:48-53. doi: 10.1016/j.prostaglandins.2018.09.008. Epub 2018 Sep 29. Prostaglandins Other Lipid Mediat. 2018. PMID: 30278231 Free PMC article. Review.
Cited by
-
Carnosinylation of Cardiac Antigens Attenuates Immunogenic Responses and Improves Function in Failing Hearts.bioRxiv [Preprint]. 2025 Aug 31:2025.08.22.671840. doi: 10.1101/2025.08.22.671840. bioRxiv. 2025. PMID: 40909674 Free PMC article. Preprint.
-
Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases.Inflammopharmacology. 2023 Feb;31(1):89-117. doi: 10.1007/s10787-022-01086-9. Epub 2022 Dec 6. Inflammopharmacology. 2023. PMID: 36471190 Free PMC article. Review.
-
Fibroblasts and immune cells: at the crossroad of organ inflammation and fibrosis.Am J Physiol Heart Circ Physiol. 2024 Feb 1;326(2):H303-H316. doi: 10.1152/ajpheart.00545.2023. Epub 2023 Dec 1. Am J Physiol Heart Circ Physiol. 2024. PMID: 38038714 Free PMC article. Review.
-
Estrogen Receptor-β Agonists Modulate T-Lymphocyte Activation and Ameliorate Left Ventricular Remodeling During Chronic Heart Failure.Circ Heart Fail. 2022 Jul;15(7):e008997. doi: 10.1161/CIRCHEARTFAILURE.121.008997. Epub 2022 Jun 22. Circ Heart Fail. 2022. PMID: 35730443 Free PMC article.
-
New Progress in the Molecular Regulations and Therapeutic Applications in Cardiac Oxidative Damage Caused by Pressure Overload.Antioxidants (Basel). 2022 Apr 29;11(5):877. doi: 10.3390/antiox11050877. Antioxidants (Basel). 2022. PMID: 35624741 Free PMC article. Review.
References
-
- Salvador AM, Nevers T, Velazquez F, Aronovitz M, Wang B, Abadia Molina A, Jaffe IZ, Karas RH, Blanton RM and Alcaide P. Intercellular Adhesion Molecule 1 Regulates Left Ventricular Leukocyte Infiltration, Cardiac Remodeling, and Function in Pressure Overload-Induced Heart Failure. J Am Heart Assoc. 2016;5:e003126. - PMC - PubMed
-
- Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A and Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

