Despite increasing aldosterone, elevated potassium is not necessary for activating aldosterone-sensitive HSD2 neurons or sodium appetite
- PMID: 33463885
- PMCID: PMC7814482
- DOI: 10.14814/phy2.14714
Despite increasing aldosterone, elevated potassium is not necessary for activating aldosterone-sensitive HSD2 neurons or sodium appetite
Abstract
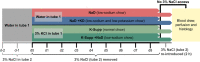
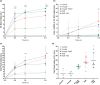
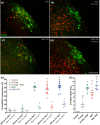
Restricting dietary sodium promotes sodium appetite in rats. Prolonged sodium restriction increases plasma potassium (pK), and elevated pK is largely responsible for a concurrent increase in aldosterone, which helps promote sodium appetite. In addition to increasing aldosterone, we hypothesized that elevated potassium directly influences the brain to promote sodium appetite. To test this, we restricted dietary potassium in sodium-deprived rats. Potassium restriction reduced pK and blunted the increase in aldosterone caused by sodium deprivation, but did not prevent sodium appetite or the activation of aldosterone-sensitive HSD2 neurons. Conversely, supplementing potassium in sodium-deprived rats increased pK and aldosterone, but did not increase sodium appetite or the activation of HSD2 neurons relative to potassium restriction. Supplementing potassium without sodium deprivation did not significantly increase aldosterone and HSD2 neuronal activation and only modestly increased saline intake. Overall, restricting dietary sodium activated the HSD2 neurons and promoted sodium appetite across a wide range of pK and aldosterone, and saline consumption inactivated the HSD2 neurons despite persistent hyperaldosteronism. In conclusion, elevated potassium is important for increasing aldosterone, but it is neither necessary nor sufficient for activating HSD2 neurons and increasing sodium appetite.
Keywords: aldosterone; dietary sodium; mineralocorticoid; potassium; salt appetite; salt hunger.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None of the authors have any conflict of interest related to this work.
Figures


References
-
- Adam, W. R. , & Dawborn, J. K. (1972). Effect of potassium depletion on mineral appetite in the rat. Journal of Comparative and Physiological Psychology, 78, 51–58. - PubMed
-
- Aguilera, G. , & Catt, K. J. (1986). Participation of voltage‐dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology, 118, 112–118. - PubMed
-
- Blake, W. D. , & Jurf, A. N. (1968). Increased voluntary Na intake in K deprived rats. Communications in Behavioral Biology Part A, 1, 107.
-
- Broadwell, R. D. , & Sofroniew, M. V. (1993). Serum proteins bypass the blood‐brain fluid barriers for extracellular entry to the central nervous system. Experimental Neurology, 120, 245–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

