Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites
- PMID: 33463911
- PMCID: PMC7814497
- DOI: 10.14814/phy2.14719
Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites
Abstract
Background: Lactic acid bacteria are commensal members of the gut microbiota and are postulated to promote host health. Secreted factors and cell surface components from Lactobacillus species have been shown to modulate the host immune system. However, the precise role of L. reuteri secreted factors and surface proteins in influencing dendritic cells (DCs) remains uncharacterized.
Hypothesis: We hypothesize that L. reuteri secreted factors will promote DC maturation, skewing cells toward an anti-inflammatory phenotype. In acute colitis, we speculate that L. reuteri promotes IL-10 and dampens pro-inflammatory cytokine production, thereby improving colitis.
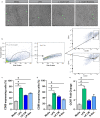
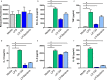
Methods & results: Mouse bone marrow-derived DCs were differentiated into immature dendritic cells (iDCs) via IL-4 and GM-CSF stimulation. iDCs exposed to L. reuteri secreted factors or UV-irradiated bacteria exhibited greater expression of DC maturation markers CD83 and CD86 by flow cytometry. Additionally, L. reuteri stimulated DCs exhibited phenotypic maturation as denoted by cytokine production, including anti-inflammatory IL-10. Using mouse colonic organoids, we found that the microinjection of L. reuteri secreted metabolites and UV-irradiated bacteria was able to promote IL-10 production by DCs, indicating potential epithelial-immune cross-talk. In a TNBS-model of acute colitis, L. reuteri administration significantly improved histological scoring, colonic cytokine mRNA, serum cytokines, and bolstered IL-10 production.
Conclusions: Overall these data demonstrate that both L. reuteri secreted factors and its bacterial components are able to promote DC maturation. This work points to the specific role of L. reuteri in modulating intestinal DCs.
New & noteworthy: Lactobacillus reuteri colonizes the mammalian gastrointestinal tract and exerts beneficial effects on host health. However, the mechanisms behind these effects have not been fully explored. In this article, we identified that L. reuteri ATTC PTA 6475 metabolites and surface components promote dendritic cell maturation and IL-10 production. In acute colitis, we also demonstrate that L. reuteri can promote IL-10 and suppress inflammation. These findings may represent a crucial mechanism for maintaining intestinal immune homeostasis.
Keywords: Lactobacillus; cytokines; dendritic cells; inflammation; metabolites.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
JV receives unrestricted research support from the Swedish Probiotic Company BioGaia AB. JV serves on the scientific advisory boards of Seed, a USA‐ based probiotics/prebiotics company, Biomica, an Israeli informatics enterprise and Plexus Worldwide, a USA‐based nutrition company. All other authors have no relationships to disclose.
Figures



References
-
- Ahrne, S. , Nobaek, S. , Jeppsson, B. , Adlerberth, I. , Wold, A. E. , & Molin, G. (1998). The normal Lactobacillus flora of healthy human rectal and oral mucosa. Journal of Applied Microbiology, 85, 88–94. - PubMed
-
- Aleljung, P. , Shen, W. , Rozalska, B. , Hellman, U. , Ljungh, A. , & Wadstrom, T. (1994). Purification of collagen‐binding proteins of Lactobacillus reuteri NCIB 11951. Current Microbiology, 28, 231–236. - PubMed
-
- Atarashi, K. , Tanoue, T. , Shima, T. , Imaoka, A. , Kuwahara, T. , Momose, Y. , Cheng, G. , Yamasaki, S. , Saito, T. , Ohba, Y. , Taniguchi, T. , Takeda, K. , Hori, S. , Ivanov, I. I. , Umesaki, Y. , Itoh, K. , & Honda, K. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 331, 337–341. - PMC - PubMed
-
- Banchereau, J. , Briere, F. , Caux, C. , Davoust, J. , Lebecque, S. , Liu, Y. J. , Pulendran, B. , & Palucka, K. (2000). Immunobiology of dendritic cells. Annual Review of Immunology, 18, 767–811. - PubMed
-
- Braat, H. , de Jong, E. C. , van den Brande, J. M. , Kapsenberg, M. L. , Peppelenbosch, M. P. , van Tol, E. A. , & van Deventer, S. J. (2004). Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. Journal of Molecular Medicine (Berl), 82, 197–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

