Choice of cystometric technique impacts detrusor contractile dynamics in wistar rats
- PMID: 33463913
- PMCID: PMC7814486
- DOI: 10.14814/phy2.14724
Choice of cystometric technique impacts detrusor contractile dynamics in wistar rats
Abstract
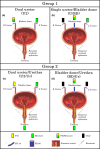
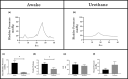
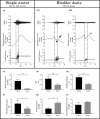
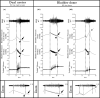
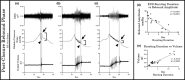
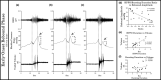
The objective of the current animal study was to investigate factors contributing to the different phases of the cystometrogram (CMG) in order to address disparities in research data reported in the current literature. Three experiments in 20 female Wistar rats were designed to investigate (1) the effects of anesthesia on the contractile pattern of the bladder during micturition; (2) the impact of the physical characteristics of the CMG technique upon the accuracy of intra-vesical pressure recordings; and (3) identification of physiological and methodological factors associated with the emptying and rebound phases during CMG. Variables tested included awake versus urethane-anesthetized conditions, use of a single catheter for both filling and intra-vesical pressure (Pves) recording versus a separate two catheter approach, and comparisons between ureter, bladder dome, and urethral catheter placements. Both awake and anesthetized conditions contributed to variations in the shape and magnitude of the CMG pressure curves. In addition, catheter size, acute incision of the bladder dome for catheter placement, use of the same catheter for filling and Pves recordings, as well as the placement and positioning of the tubing, all contributed to alterations of the physiological properties and characteristic of the various CMG phases, including the frequent occurrence of an artificial rebound during the third phase of micturition. The present results demonstrate how different experimental conditions lead not only to variability in Pves curves, but consistency of the measurements as well, which needs to be accounted for when interpreting CMG outcome data.
Keywords: bladder; cystometrogram; external urethral sphincter; micturition; urodynamics.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures

References
-
- Andersson, K.‐E. , Soler, R. , & Füllhase, C. (2011). Rodent models for urodynamic investigation. Neurourology and Urodynamics, 30(5), 636–646. - PubMed
-
- Birder, L. A. (2006). Urinary bladder urothelium: Molecular sensors of chemical/thermal/mechanical stimuli. Ascular Pharmacology, 45(4), 221–226. - PubMed
-
- Birder, L. , Kullmann, F. A. , Lee, H. , Barrick, S. , de Groat, W. , Kanai, A. , & Caterina, M. (2007). Activation of urothelial transient receptor potential vanilloid 4 by 4α‐phorbol 12,13‐didecanoate contributes to altered bladder reflexes in the rat. Journal of Pharmacology and Experimental Therapeutics, 323(1), 227–235. - PubMed
-
- Brantley, S. F. , Quesada, E. M. , & David, C. (1964). Studies on the dynamics of micturition: Observations on healthy men*. Journal of Urology, 92(5), 455–463. - PubMed
-
- Cannon, T. W. , & Damaser, M. S. (2001). Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sciences, 69, 1193–1202. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

