Molecular Imaging Approaches in Dementia
- PMID: 33464184
- PMCID: PMC7924525
- DOI: 10.1148/radiol.2020200028
Molecular Imaging Approaches in Dementia
Abstract
The increasing prevalence of dementia worldwide places a high demand on healthcare providers to perform a diagnostic work-up in relatively early stages of the disease, given that the pathologic process usually begins decades before symptoms are evident. Structural imaging is recommended to rule out other disorders and can only provide diagnosis in a late stage with limited specificity. Where PET imaging previously focused on the spatial pattern of hypometabolism, the past decade has seen the development of novel tracers to demonstrate characteristic protein abnormalities. Molecular imaging using PET/SPECT is able to show amyloid and tau deposition in Alzheimer disease and dopamine depletion in parkinsonian disorders starting decades before symptom onset. Novel tracers for neuroinflammation and synaptic density are being developed to further unravel the molecular pathologic characteristics of dementia disorders. In this article, the authors review the current status of established and emerging PET tracers in a diagnostic setting and also their value as prognostic markers in research studies and outcome measures for clinical trials in Alzheimer disease.
© RSNA, 2021.
Figures
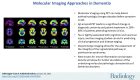




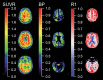
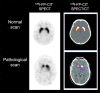
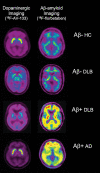

References
-
- Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15(5):455–532. - PubMed
-
- Ten Kate M, Barkhof F, Boccardi M, et al. Clinical validity of medial temporal atrophy as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:167–182.e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

