Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke: The SKIP Randomized Clinical Trial
- PMID: 33464334
- PMCID: PMC7816103
- DOI: 10.1001/jama.2020.23522
Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke: The SKIP Randomized Clinical Trial
Erratum in
-
Incorrect P Value and Figure Label Error.JAMA. 2021 May 4;325(17):1795. doi: 10.1001/jama.2021.5454. JAMA. 2021. PMID: 33944890 Free PMC article. No abstract available.
Abstract
Importance: Whether intravenous thrombolysis is needed in combination with mechanical thrombectomy in patients with acute large vessel occlusion stroke is unclear.
Objective: To examine whether mechanical thrombectomy alone is noninferior to combined intravenous thrombolysis plus mechanical thrombectomy for favorable poststroke outcome.
Design, setting, and participants: Investigator-initiated, multicenter, randomized, open-label, noninferiority clinical trial in 204 patients with acute ischemic stroke due to large vessel occlusion enrolled at 23 hospital networks in Japan from January 1, 2017, to July 31, 2019, with final follow-up on October 31, 2019.
Interventions: Patients were randomly assigned to mechanical thrombectomy alone (n = 101) or combined intravenous thrombolysis (alteplase at a 0.6-mg/kg dose) plus mechanical thrombectomy (n = 103).
Main outcomes and measures: The primary efficacy end point was a favorable outcome defined as a modified Rankin Scale score (range, 0 [no symptoms] to 6 [death]) of 0 to 2 at 90 days, with a noninferiority margin odds ratio of 0.74, assessed using a 1-sided significance threshold of .025 (97.5% CI). There were 7 prespecified secondary efficacy end points, including mortality by day 90. There were 4 prespecified safety end points, including any intracerebral hemorrhage and symptomatic intracerebral hemorrhage within 36 hours.
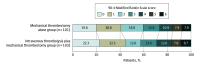
Results: Among 204 patients (median age, 74 years; 62.7% men; median National Institutes of Health Stroke Scale score, 18), all patients completed the trial. Favorable outcome occurred in 60 patients (59.4%) in the mechanical thrombectomy alone group and 59 patients (57.3%) in the combined intravenous thrombolysis plus mechanical thrombectomy group, with no significant between-group difference (difference, 2.1% [1-sided 97.5% CI, -11.4% to ∞]; odds ratio, 1.09 [1-sided 97.5% CI, 0.63 to ∞]; P = .18 for noninferiority). Among the 7 secondary efficacy end points and 4 safety end points, 10 were not significantly different, including mortality at 90 days (8 [7.9%] vs 9 [8.7%]; difference, -0.8% [95% CI, -9.5% to 7.8%]; odds ratio, 0.90 [95% CI, 0.33 to 2.43]; P > .99). Any intracerebral hemorrhage was observed less frequently in the mechanical thrombectomy alone group than in the combined group (34 [33.7%] vs 52 [50.5%]; difference, -16.8% [95% CI, -32.1% to -1.6%]; odds ratio, 0.50 [95% CI, 0.28 to 0.88]; P = .02). Symptomatic intracerebral hemorrhage was not significantly different between groups (6 [5.9%] vs 8 [7.7%]; difference, -1.8% [95% CI, -9.7% to 6.1%]; odds ratio, 0.75 [95% CI, 0.25 to 2.24]; P = .78).
Conclusions and relevance: Among patients with acute large vessel occlusion stroke, mechanical thrombectomy alone, compared with combined intravenous thrombolysis plus mechanical thrombectomy, failed to demonstrate noninferiority regarding favorable functional outcome. However, the wide confidence intervals around the effect estimate also did not allow a conclusion of inferiority.
Trial registration: umin.ac.jp/ctr Identifier: UMIN000021488.
Conflict of interest statement
Figures
Comment in
-
Intravenous Thrombolysis Before Endovascular Thrombectomy for Acute Ischemic Stroke.JAMA. 2021 Jan 19;325(3):229-231. doi: 10.1001/jama.2020.22388. JAMA. 2021. PMID: 33464293 No abstract available.
-
Functional Outcomes Among Patients With Acute Ischemic Stroke After Mechanical Thrombectomy With or Without Intravenous Thrombolysis.JAMA. 2021 May 18;325(19):2019-2020. doi: 10.1001/jama.2021.4034. JAMA. 2021. PMID: 34003229 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

