Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial
- PMID: 33464336
- PMCID: PMC7816106
- DOI: 10.1001/jama.2020.25864
Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial
Abstract
Importance: It is unknown whether angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) have a positive, neutral, or negative effect on clinical outcomes in patients with coronavirus disease 2019 (COVID-19).
Objective: To determine whether discontinuation compared with continuation of ACEIs or ARBs changed the number of days alive and out of the hospital through 30 days.
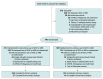
Design, setting, and participants: A randomized clinical trial of 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization (enrolled: April 9-June 26, 2020; final follow-up: July 26, 2020).
Interventions: Discontinuation (n = 334) or continuation (n = 325) of ACEIs or ARBs.
Main outcomes and measures: The primary outcome was the number of days alive and out of the hospital through 30 days. Secondary outcomes included death, cardiovascular death, and COVID-19 progression.
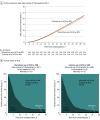
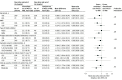
Results: Among 659 patients, the median age was 55.1 years (interquartile range [IQR], 46.1-65.0 years), 14.7% were aged 70 years or older, 40.4% were women, and 100% completed the trial. The median time from symptom onset to hospital admission was 6 days (IQR, 4-9 days) and 27.2% of patients had an oxygen saturation of less than 94% of room air at baseline. In terms of clinical severity, 57.1% of patients were considered mild at hospital admission and 42.9% were considered moderate. There was no significant difference in the number of days alive and out of the hospital in patients in the discontinuation group (mean, 21.9 days [SD, 8 days]) vs patients in the continuation group (mean, 22.9 days [SD, 7.1 days]) and the mean ratio was 0.95 (95% CI, 0.90-1.01). There also was no statistically significant difference in death (2.7% for the discontinuation group vs 2.8% for the continuation group; odds ratio [OR], 0.97 [95% CI, 0.38-2.52]), cardiovascular death (0.6% vs 0.3%, respectively; OR, 1.95 [95% CI, 0.19-42.12]), or COVID-19 progression (38.3% vs 32.3%; OR, 1.30 [95% CI, 0.95-1.80]). The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs 7.7% in the continuation group), shock requiring vasopressors (8.4% vs 7.1%, respectively), acute myocardial infarction (7.5% vs 4.6%), new or worsening heart failure (4.2% vs 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs 2.8%).
Conclusions and relevance: Among patients hospitalized with mild to moderate COVID-19 and who were taking ACEIs or ARBs before hospital admission, there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue vs continue these medications. These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT04364893.
Conflict of interest statement
Figures
Comment in
-
Brugada syndrome and reduced right ventricular outflow tract conduction reserve: a final common pathway?Eur Heart J. 2021 Mar 14;42(11):1073-1081. doi: 10.1093/eurheartj/ehaa1051. Eur Heart J. 2021. PMID: 33421051
-
A randomized trial supports the recommendation to continue treatment with ACEi or ARBs during hospitalization for COVID-19.Eur Heart J. 2021 Mar 14;42(11):1061-1062. doi: 10.1093/eurheartj/ehab106. Eur Heart J. 2021. PMID: 33659979 Free PMC article. No abstract available.
-
From confusion to clarity: RAS blockade in patients hospitalized with COVID-19.Kidney Int. 2021 May;99(5):1059-1061. doi: 10.1016/j.kint.2021.02.034. Epub 2021 Mar 19. Kidney Int. 2021. PMID: 33753072 Free PMC article. No abstract available.
References
-
- Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. JAMA. 2020;323(18):1769-1770. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

