Nanobody: a promising toolkit for molecular imaging and disease therapy
- PMID: 33464410
- PMCID: PMC7815856
- DOI: 10.1186/s13550-021-00750-5
Nanobody: a promising toolkit for molecular imaging and disease therapy
Abstract
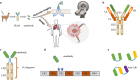
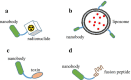
Nanobodies are the recombinant variable domains of heavy-chain-only antibodies, with many unique properties such as small size, excellent solubility, superior stability, quick clearance from blood, and deep tissue penetration. As a result, nanobodies have become a promising tool for the diagnosis and therapy of diseases. As imaging tracers, nanobodies allow an early acquisition of high-quality images, provide a comprehensive evaluation of the disease, and subsequently enable a personalized precision therapy. As therapeutic agents, nanobodies enable a targeted therapy by lesion-specific delivery of drugs and effector domains, thereby improving the specificity and efficacy of the therapy. Up to date, a wide variety of nanobodies have been developed for a broad range of molecular targets and have played a significant role in patients with a broad spectrum of diseases. In this review, we aim to outline the current state-of-the-art research on the nanobodies for medical applications and then discuss the challenges and strategies for their further clinical translation.
Keywords: Cancer; Inflammation; Molecular imaging; Nanobody; Therapy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

