Evidence for variable chlorophyll fluorescence of photosystem I in vivo
- PMID: 33464442
- PMCID: PMC8382641
- DOI: 10.1007/s11120-020-00814-y
Evidence for variable chlorophyll fluorescence of photosystem I in vivo
Abstract
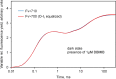
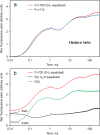
Room temperature fluorescence in vivo and its light-induced changes are dominated by chlorophyll a fluorescence excited in photosystem II, F(II), peaking around 685 nm. Photosystem I fluorescence, F(I), peaking around 730 nm, so far has been assumed to be constant in vivo. Here, we present evidence for significant contributions of F(I) to variable fluorescence in the green unicellular alga Chlorella vulgaris, the cyanobacterium Synechococcus leopoliensis and a light-green ivy leaf. A Multi-Color-PAM fluorometer was applied for measurements of the polyphasic fluorescence rise (O-I1-I2-P) induced by strong 440 nm light in a dilute suspension of Chlorella, with detection alternating between emission above 700 nm (F > 700) and below 710 nm (F < 710). By averaging 10 curves each of the F > 700 and F < 710 recordings even small differences could be reliably evaluated. After equalizing the amplitudes of the O-I1 phase, which constitutes a specific F(II) response, the O-I1-I2 parts of the two recordings were close to identical, whereas the I2-P phase was larger in F > 700 than in F < 710 by a factor of 1.42. In analogous measurements with Synechococcus carried out in the dark state 2 using strong 625 nm actinic light, after O-I1 equalization the I2-P phase in F > 700 exceeded that in F < 710 even by a factor of 1.99. In measurements with Chlorella, the I2-P phase and with it the apparent variable fluorescence of PS I, Fv(I), were suppressed by moderate actinic background light and by the plastoquinone antagonist DBMIB. Analogous measurements with leaves are rendered problematic by unavoidable light intensity gradients and the resulting heterogenic origins of F > 700 and F < 710. However, a light-green young ivy leaf gave qualitatively similar results as those obtained with the suspensions, thus strongly suggesting the existence of Fv(I) also in leaves.
Keywords: Chlorella; F > 700 nm; MULTI-COLOR-PAM; Polyphasic fluorescence rise O-I 1-I 2-P; Room temperature fluorescence emission; Synechococcus leopoliensis.
© 2021. The Author(s).
Conflict of interest statement
On behalf of both authors, the corresponding author states that there is no conflict of interest.
Figures









References
-
- Briantais J-M, Vernotte C, Krause GH, Weis E. Chlorophyll a fluorescence of higher plants: chloroplasts and leaves. In: Govindjee G, Amesz J, Fork DJ, editors. Light emission by plants and bacteria. New York: Academic Press; 1986. pp. 539–583.
-
- Byrdin M, Rimke I, Schlodder E, Stehlik D, Roelofs TA. Decay kinetics and quantum yields of fluorescence in photosystem I from Synechococcus elongatus with P700 in the reduced and oxidized state:are the kinetics of excited state decay trap-limited or transfer-limited? Biophys J. 2000;79:992–1007. doi: 10.1016/S0006-3495(00)76353-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

