Bilateral 0.19 mg Fluocinolone Acetonide Intravitreal Implant in the Successful Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis and Secondary Macular Oedema: A Case Report and Review of Intravitreal Therapies
- PMID: 33464558
- PMCID: PMC7887104
- DOI: 10.1007/s40123-020-00328-9
Bilateral 0.19 mg Fluocinolone Acetonide Intravitreal Implant in the Successful Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis and Secondary Macular Oedema: A Case Report and Review of Intravitreal Therapies
Abstract
Introduction: Treatment of juvenile idiopathic arthritis (JIA)-associated uveitis necessitates the use of long-term corticosteroids or immunosuppressive agents, each of which poses their own significant side effect profile. Initial treatment requires intensive topical glucocorticoids, with a step-up approach employing immunosuppressive agents for those cases with poor response or high-risk complications such as macular oedema. To date, there is minimal evidence to support a specific approach to such complicated subgroups. We present the first case to successfully employ the 0.19 mg fluocinolone acetonide implant (ILUVIEN®, Alimera Sciences, Hampshire, UK) as a novel device for prolonged intravitreal administration of disease-modifying agents for patients with JIA complicated by uveitis.
Methods: This retrospective case report describes a 20-year old woman diagnosed with oligoarticular JIA complicated by chronic uveitis and associated cystoid macular oedema (CMO). Considering factors including the patient's non-compliance, age, lens status, non-steroid response, and good response to short-term intravitreal steroid therapy, the 0.19 mg fluocinolone acetonide intravitreal implant was deemed an appropriate step-up treatment option.
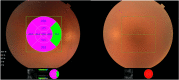
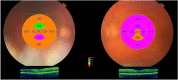
Results: At 12-month follow-up, the left eye (OS) showed an improvement in visual acuity to 6/15 - 1 from 6/60 + 1 (0.42 from 0.98 logMAR) (pre-insertion) and a reduction in central retinal thickness (CRT) of 199 μm from 471 μm. The right eye (OD), treated 10 months later, showed an improvement in visual acuity to 6/7.5 from 6/24 - 1 (0.10 from 0.56 LogMAR) and a reduction in CRT of 327 μm 6 months after treatment.
Conclusion: In this case, the 0.19 mg fluocinolone acetonide implant provided safe and effective long-term treatment of JIA-associated uveitis and secondary CMO. This potentially offers an alternative approach to complex cases that show good response to short-term corticosteroid use.
Keywords: Cystoid macular oedema; Fluocinolone acetonide; JIA; Uveitis.
Figures

Similar articles
-
BILATERAL INTRAVITREAL 0.19-MG FLUOCINOLONE ACETONIDE IMPLANT FOR PERSISTENT NONDIABETIC CYSTOID MACULAR EDEMA AFTER VITRECTOMY.Retin Cases Brief Rep. 2021 May 1;15(3):261-265. doi: 10.1097/ICB.0000000000000779. Retin Cases Brief Rep. 2021. PMID: 30015770
-
Fluocinolone acetonide intravitreal implant (Retisert® ) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis.Acta Ophthalmol. 2018 Sep;96(6):648-651. doi: 10.1111/aos.13744. Epub 2018 Apr 14. Acta Ophthalmol. 2018. PMID: 29655222
-
Fluocinolone Acetonide 0.19 mg Implant in Patients with Cystoid Macular Edema Due To Irvine-Gass Syndrome.Int Med Case Rep J. 2021 Feb 26;14:127-132. doi: 10.2147/IMCRJ.S295045. eCollection 2021. Int Med Case Rep J. 2021. PMID: 33664598 Free PMC article.
-
Preventing relapse in non-infectious uveitis affecting the posterior segment of the eye - evaluating the 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN®).J Ophthalmic Inflamm Infect. 2020 Nov 30;10(1):32. doi: 10.1186/s12348-020-00225-z. J Ophthalmic Inflamm Infect. 2020. PMID: 33251553 Free PMC article. Review.
-
Fluocinolone Acetonide Intravitreal Implant 0.19 mg (ILUVIEN®): A Review in Diabetic Macular Edema.Drugs. 2017 Apr;77(5):575-583. doi: 10.1007/s40265-017-0722-4. Drugs. 2017. PMID: 28283896 Review.
Cited by
-
The double-edged sword of inflammation in inherited retinal degenerations: Clinical and preclinical evidence for mechanistically and prognostically impactful but treatable complications.Front Cell Dev Biol. 2023 Apr 13;11:1177711. doi: 10.3389/fcell.2023.1177711. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37123408 Free PMC article.
-
Fluocinolone acetonide 0.2 µg/day intravitreal implant in non-infectious uveitis affecting the posterior segment: EU expert user panel consensus-based clinical recommendations.J Ophthalmic Inflamm Infect. 2024 May 30;14(1):22. doi: 10.1186/s12348-024-00402-4. J Ophthalmic Inflamm Infect. 2024. PMID: 38814386 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

