The spleen: "epicenter" in malaria infection and immunity
- PMID: 33464668
- PMCID: PMC8518401
- DOI: 10.1002/JLB.4RI1020-713R
The spleen: "epicenter" in malaria infection and immunity
Abstract
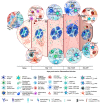
The spleen is a complex secondary lymphoid organ that plays a crucial role in controlling blood-stage infection with Plasmodium parasites. It is tasked with sensing and removing parasitized RBCs, erythropoiesis, the activation and differentiation of adaptive immune cells, and the development of protective immunity, all in the face of an intense inflammatory environment. This paper describes how these processes are regulated following infection and recognizes the gaps in our current knowledge, highlighting recent insights from human infections and mouse models.
Keywords: Ab; B cells; T cells; cytokines; inflammation.
© 2021 The Authors. Journal of Leukocyte Biology published by Wiley Periodicals LLC on behalf of Society for Leukocyte Biology.
Figures
References
-
- Steiniger B, Barth P. Microanatomy and function of the spleen. Adv Anat Embryol Cell Biol. 2000;151:III‐IX. 1–101. - PubMed
-
- Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/β‐haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269‐271. - PubMed
-
- Ridley RG, Dorn A, Matile H, Kansy M. Haem polymerization in malaria. Nature. 1995;378:138‐139. - PubMed
-
- Egan TJ, Ross DC, Adams PA. Quinoline anti‐malarial drugs inhibit spontaneous formation of β‐haematin (malaria pigment). FEBS Lett. 1994;352:54‐57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

