Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review
- PMID: 33464677
- PMCID: PMC8248154
- DOI: 10.1111/dom.14322
Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review
Abstract
Aims: To conduct a systematic literature review to identify recent epidemiological, biomarker, genetic and clinical evidence that expands our understanding of nonalcoholic fatty liver disease (NAFLD) as a metabolic disorder.
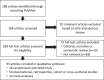
Materials and methods: We performed a literature search using PubMed to identify trials, observational studies and meta-analyses published in the past 5 years.
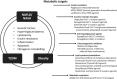
Results: A total of 95 publications met prespecified inclusion criteria and reported on the interplay between NAFLD/nonalcoholic steatohepatitis (NASH) and metabolic dysfunction, in terms of disease burden and/or epidemiology (n = 10), pathophysiology, risk factors and associated conditions (n = 29), diagnosis and biomarkers (n = 34), and treatment approaches (n = 22). There is a growing body of evidence on the links between NAFLD/NASH pathogenesis and mechanisms of metabolic dysfunction, through liver lipid accumulation, insulin resistance, inflammation, apoptosis, and fibrogenic remodelling within the liver. The frequent co-occurrence of NAFLD with obesity, metabolic syndrome and type 2 diabetes supports this premise. Therapeutic approaches originally envisaged for type 2 diabetes or obesity (such as glucagon-like peptide-1 receptor agonists, sodium-glucose co-transporter-2 inhibitors, insulin sensitizers and bariatric surgery) have shown promising signs of benefit for patients with NAFLD/NASH.
Conclusions: Given the complex interplay between NAFLD and metabolic dysfunction, there is an urgent need for multidisciplinary collaboration and established protocols for care of patients with NAFLD that are individualized and ideally support reduction of overall metabolic risk as well as treatment for NASH.
Keywords: GLP-1; fatty liver disease; insulin resistance; pharmaco-epidemiology; type 2 diabetes.
© 2021 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
B. C. reports grants and personal fees from Amgen, Regeneron and Sanofi, and personal fees from Abbott, Akcea, AstraZeneca, Bristol Myers Squibb, Genfit, Gilead, Eli Lilly and Company, Merck (MSD) and Novo Nordisk. R. L. serves as a consultant or advisory board member for 89bio, Alnylam, Arrowhead Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cirius, CohBar, DiCerna, Galmed, Gilead, Glympse bio, Intercept, Ionis, Metacrine, NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Sagimet and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Novartis, Pfizer, pH Pharma and Siemens. He is also co‐founder of Liponexus, Inc. A. J. S. is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect Inversago and Galmed. He has served as a consultant to AstraZeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Janssen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento and Surrozen. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers Squibb, Shire, Intercept, Merck, AstraZeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate. C. D. B. discloses no conflicts of interest.
Figures
References
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328‐357. - PubMed
-
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388‐1402. - PubMed
-
- Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(4 Suppl):64‐70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR002649/TR/NCATS NIH HHS/United States
- U01 DK061731/DK/NIDDK NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- U01 AA026979/AA/NIAAA NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- U01 AA026966/AA/NIAAA NIH HHS/United States
- UH2 AA026971/AA/NIAAA NIH HHS/United States
- R01 DK124318/DK/NIDDK NIH HHS/United States
- R21 CA183954/CA/NCI NIH HHS/United States
- R01 DK121378/DK/NIDDK NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
- KL2 TR002648/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

