Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial
- PMID: 33464703
- PMCID: PMC8247845
- DOI: 10.1111/dom.14319
Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial
Abstract
Aims: To investigate the potential synergistic effects of combined exenatide (EXE) and dapagliflozin (DAPA) versus (PLAC) placebo and DAPA on hepatocellular lipid (HCL) reduction after 24 weeks of treatment.
Materials and methods: Thirty patients with type 2 diabetes were randomized to weekly EXE and daily DAPA (n = 16) or weekly PLAC and daily DAPA (n = 14). Inclusion criteria were glycated haemoglobin (HbA1c) 48 to 97 mmol/mol (6.5-11%), age 18 to 75 years, body mass index (BMI) ≥25 kg/m2 and metformin ≥1000 mg. The primary endpoint, HCL levels, were measured at baseline and after 24 weeks of treatment using magnetic resonance spectroscopy. Between-group effects were analysed using general linear models, adjusted for baseline outcome variables, age, sex and BMI. Within-group differences were assessed using a paired t-test.
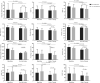
Results: After 24 weeks, HCLs were reduced in both treatment groups (absolute change from baseline: EXE + DAPA -4.4%, 95% confidence interval [CI] -8.2, -0.7, P < 0.05; PLAC + DAPA -3.9%, 95% CI -6.0, -1.7, P < 0.01; relative change: EXE + DAPA -35.6%, PLAC + DAPA -32.3%) with no difference between groups. Similar findings were observed for subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). HbA1c (EXE + DAPA -17.8 mmol/mol, [95% CI -24.8, -10.8], P <0.001; PLAC + DAPA -6.9 mmol/mol, [95% CI -10.5, -3.3], P = 0.001) and fasting glucose significantly decreased in both groups, although EXE + DAPA achieved better glycaemic control than PLAC + DAPA (adjusted difference: HbA1c -6.0 mmol/mol [95% CI -9.7, -2.2], P < 0.01). Body weight was reduced in both treatment groups (EXE + DAPA -7.3 kg, 95% CI -9.9, -4.8, P <0.001; PLAC + DAPA -4.6 kg, 95% CI -7.4, -1.8, P <0.01) with comparable results between groups. Changes in HCLs and weight, hip and waist circumference, VAT and SAT were positively associated.
Conclusion: After 24 weeks, HCLs were significantly but comparably reduced in the EXE + DAPA and PLAC + DAPA groups, despite significantly better glycaemic control in the combined group EXE + DAPA. Changes in HCLs were associated with weight loss and reduction of visceral adiposity, but not with glucose control. Further studies are necessary to evaluate possible additional long-term effects of a combined treatment.
Keywords: CVD; GLP-1 receptor agonist; NAFLD; SGLT2 inhibitor; ectopic lipids; glycaemic control; magnetic resonance imaging; metabolic syndrome; obesity; prevention; type 2 diabetes mellitus; weight loss.
© 2021 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial.Lancet Diabetes Endocrinol. 2016 Dec;4(12):1004-1016. doi: 10.1016/S2213-8587(16)30267-4. Epub 2016 Sep 16. Lancet Diabetes Endocrinol. 2016. PMID: 27651331 Clinical Trial.
-
Fixed-Dose Combination of Dapagliflozin + Sitagliptin + Metformin in Patients with Type 2 Diabetes Poorly Controlled with Metformin: Phase 3, Randomized Comparison with Dual Combinations.Adv Ther. 2023 Jul;40(7):3227-3246. doi: 10.1007/s12325-023-02523-z. Epub 2023 May 31. Adv Ther. 2023. PMID: 37258803 Clinical Trial.
-
Long-term effects of dapagliflozin plus saxagliptin versus glimepiride on a background of metformin in patients with type 2 diabetes: Results of a 104-week extension to a 52-week randomized, phase 3 study and liver fat MRI substudy.Diabetes Obes Metab. 2022 Jan;24(1):61-71. doi: 10.1111/dom.14548. Epub 2021 Sep 28. Diabetes Obes Metab. 2022. PMID: 34514692 Free PMC article. Clinical Trial.
-
The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta-analysis of randomised controlled trials.BMJ Open. 2014 Apr 7;4(4):e004619. doi: 10.1136/bmjopen-2013-004619. BMJ Open. 2014. PMID: 24710132 Free PMC article. Review.
-
Additional health education and nutrition management cause more weight loss than concurrent training in overweight young females.Complement Ther Clin Pract. 2023 May;51:101721. doi: 10.1016/j.ctcp.2023.101721. Epub 2023 Jan 17. Complement Ther Clin Pract. 2023. PMID: 36669325 Review.
Cited by
-
Biomarkers for Assessing Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus on Sodium-Glucose Cotransporter 2 Inhibitor Therapy.J Clin Med. 2023 Oct 16;12(20):6561. doi: 10.3390/jcm12206561. J Clin Med. 2023. PMID: 37892698 Free PMC article. Review.
-
Effect of glucagon-like peptide-1 receptor agonists on fat distribution in patients with type 2 diabetes: A systematic review and meta-analysis.J Diabetes Investig. 2022 Jul;13(7):1149-1160. doi: 10.1111/jdi.13775. Epub 2022 Mar 7. J Diabetes Investig. 2022. PMID: 35191185 Free PMC article.
-
Impact of tofogliflozin on hepatic outcomes: a systematic review.Eur J Clin Pharmacol. 2023 Oct;79(10):1281-1290. doi: 10.1007/s00228-023-03537-w. Epub 2023 Jul 18. Eur J Clin Pharmacol. 2023. PMID: 37462748
-
Combination therapies in nonalcoholic fatty liver disease using antidiabetic and disease-specific drugs.Ann Gastroenterol. 2023 Jul-Aug;36(4):378-391. doi: 10.20524/aog.2023.0806. Epub 2023 May 29. Ann Gastroenterol. 2023. PMID: 37396007 Free PMC article. Review.
-
Efficacy and Safety of Tirzepatide Compared with GLP-1 RAs in Patients with Type 2 Diabetes Treated with Basal Insulin: A Network Meta-analysis.Diabetes Ther. 2025 Jun;16(6):1279-1311. doi: 10.1007/s13300-025-01728-5. Epub 2025 Apr 11. Diabetes Ther. 2025. PMID: 40214900 Free PMC article.
References
-
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71(4):793‐801. - PubMed
-
- European Association for the Study of the Liver . European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121‐1140. - PubMed
-
- Holmer M, Melum E, Isoniemi H, et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. 2018;38(11):2082‐2090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

