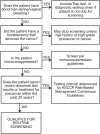
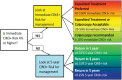
Summary of Current Guidelines for Cervical Cancer Screening and Management of Abnormal Test Results: 2016-2020
- PMID: 33464997
- PMCID: PMC8020523
- DOI: 10.1089/jwh.2020.8918
Summary of Current Guidelines for Cervical Cancer Screening and Management of Abnormal Test Results: 2016-2020
Abstract
Cervical cancer can be prevented through routine screening and follow-up of abnormal results. Several guidelines have been published in the last 4 years from various medical societies and organizations. These guidelines aim to personalize screening and management, reducing unnecessary testing in low-risk patients and managing high-risk patients with more intensive follow-up. However, the resulting complexity can lead to confusion among providers. The CDC, NCI, and obstetrician-gynecologists involved in guideline development summarized current screening and management guidelines. For screening, guidelines for average-risk and high-risk populations are summarized and presented. For management, differences between the 2012 and 2019 consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors are summarized. Current screening guidelines for average-risk individuals have minor differences, but are evolving toward an HPV-based strategy. For management, HPV testing is preferred to cytology because it is a more sensitive test for cancer precursor detection and also allows for precise risk stratification. Current risk-based screening and management strategies can improve care by reducing unnecessary tests and procedures in low-risk patients and focusing resources on high-risk patients. Knowledge of screening and management guidelines is important to improve adherence and avoid both over- and under-use of screening and colposcopy.
Keywords: HPV tests; Pap tests; cervical cancer screening; follow-up of abnormal tests.
Conflict of interest statement
R.B.P., Mona S., G.F.S., and S.F. have no conflicts of interest to disclose. Mark S. and N.W.: The National Cancer Institute has received cervical screening tests at reduced or no cost from Qiagen, Roche, BD, MobileODT, and Arbor Vita for independent evaluations of screening methods and strategies. R.L.G.: Inovio Pharmaceuticals DSMB, ASCCP Consultant.
Figures
References
-
- SEER. Cancer stat facts: Cervical cancer. Published 2013. https://seer.cancer.gov/statfacts/html/cervix.html Accessed October5, 2020
-
- Schiffman MH, Castle P. Epidemiologic studies of a necessary causal risk factor: Human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst 2003;95:E2. - PubMed
-
- Castle PE, Kinney WK, Xue X, et al. . Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: An observational cohort study. Ann Intern Med 2018;168:20–29 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials