Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts
- PMID: 33465051
- PMCID: PMC7919722
- DOI: 10.1172/JCI143645
Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts
Abstract
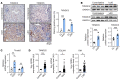
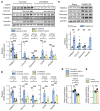
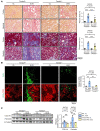
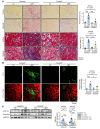
Renal fibrosis, a common pathological manifestation of virtually all types of chronic kidney disease (CKD), often results in diffuse kidney scarring and predisposes to end-stage renal disease. Currently, there is no effective therapy against renal fibrosis. Recently, our laboratory identified an ER-resident protein, thioredoxin domain containing 5 (TXNDC5), as a critical mediator of cardiac fibrosis. Transcriptome analyses of renal biopsy specimens from patients with CKD revealed marked TXNDC5 upregulation in fibrotic kidneys, suggesting a potential role of TXNDC5 in renal fibrosis. Employing multiple fluorescence reporter mouse lines, we showed that TXNDC5 was specifically upregulated in collagen-secreting fibroblasts in fibrotic mouse kidneys. In addition, we showed that TXNDC5 was required for TGF-β1-induced fibrogenic responses in human kidney fibroblasts (HKFs), whereas TXNDC5 overexpression was sufficient to promote HKF activation, proliferation, and collagen production. Mechanistically, we showed that TXNDC5, transcriptionally controlled by the ATF6-dependent ER stress pathway, mediated its profibrogenic effects by enforcing TGF-β signaling activity through posttranslational stabilization and upregulation of type I TGF-β receptor in kidney fibroblasts. Using a tamoxifen-inducible, fibroblast-specific Txndc5 knockout mouse line, we demonstrated that deletion of Txndc5 in kidney fibroblasts mitigated the progression of established kidney fibrosis, suggesting the therapeutic potential of TXNDC5 targeting for renal fibrosis and CKD.
Keywords: Cell Biology; Fibrosis; Nephrology; Protein misfolding.
Conflict of interest statement
Figures



Comment in
-
A clear pathway to tubulointerstitial disease: is an exclusive focus on fibrosis justified?J Clin Invest. 2021 Mar 1;131(5):e144803. doi: 10.1172/JCI144803. J Clin Invest. 2021. PMID: 33645547 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

